Neisseria gonorrhoeae
Neisseria gonorrhoeae (N. gonorrhoeae) causes gonorrhoea, a sexually transmitted infection (STI) with an incidence of ~190 million cases annually across all geographies. N. gonorrhoeae infection is rarely fatal. However, the pathogen causes significant morbidity through chronic, untreated infections, particularly amongst women. Whilst vaccine development remains challenging, there is new optimism amongst experts due to promising data on cross protection from the N. meningitidis vaccine. Ensuring vaccine uptake is likely to pose challenges. These result from the age of target population (young adults) and potential resistance to vaccination for an STI. However, there is a strong case for vaccine development due to the high incidence, morbidity, and urgency of AMR threat associated with N. gonorrhoeae infection.
N. gonorrhoeae falls into a cluster of pathogens for which advancing early R&D is the priority. The primary recommendation is to accelerate entry of promising pre-clinical candidates into Phase I human trials. The secondary recommendations are to focus on pre-clinical research and incentivise the development of a combination vaccine.
N. gonorrhoeae is a Gram-negative bacterium that causes sexually transmitted infections (STIs) and can affect both men and women. It can also be transmitted from mothers to children during childbirth 279. N. gonorrhoeae infection can significantly affect reproductive health and increases the risk of HIV transmission 279,280. The most common site of infection is the urogenital tract, but N. gonorrhoeae may also affect other areas of the body, causing anorectal or pharyngeal infection, and more rarely, conjunctival or ovarian infections 281.
Symptoms of gonorrhoea include vaginal discharge, vaginal bleeding, dyspareunia and abdominal/pelvic pain in women, and painful urination, pus-like discharge from the penis and testicular pain or swelling in men. However, N. gonorrhoeae infection is frequently asymptomatic, particularly in women, resulting in difficulties in obtaining a diagnosis. Untreated infections in women can result in pelvic inflammatory disease, ectopic pregnancies, infertility and chronic pelvic pain, and these complications can develop in the absence of symptoms.
N. gonorrhoeae is distributed throughout the world with the highest burden in low and middle-income countries. The incidence of gonorrhoea is particularly high in Africa and in the Western Pacific 282. The risk of contracting gonorrhoea is highest among men who have sex with men and HIV-positive individuals. Repeat infections are common; natural immunity appears to confer a limited protective effect from subsequent infection 283,284. No vaccines are currently available, but retrospective studies show some evidence of cross-protection from the MeNZB N. meningitidis vaccine 285.
Direct health impact
In this analysis, disease burden is used as a proxy for the potential direct health impact of vaccination. N. gonorrhoeae infection has a substantial direct public health impact because of its high global incidence and morbidity; however, the disease has only a low impact on mortality. According to the Institute for Health Metrics and Evaluation, there were 190 million incident cases of gonorrhoea, ~500,000 years lived with disability and 3,000 deaths in 2016 31. This source uses a defined methodology and is used in the global health community. The data can therefore be viewed with a reasonable level of confidence.
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2) and morbidity was categorised as high (score of 2 out of 2).
Secondary health impact
N. gonorrhoeae’s secondary health impact is also substantial. N. gonorrhoeae is associated with infertility in women, increased susceptibility to HIV infection 286,287, and low birth weight and pre-term birth in infected mothers 282,288.
Sub-population benefits
A vaccine targeting N. gonorrhoeae would likely have significant benefits for specific sub-populations including prevention of infertility in women and lowering the rate of transmission of HIV infection in high-risk populations including men who have sex with men.
Antibiotic use
Antibiotics are currently the only treatment for gonorrhoea infections. Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles 289. Many regimens consist of a one-time, dual therapy dose (for example, the CDC recommends ceftriaxone given as an intra-muscular injection in combination with oral azithromycin). The rationale for using single dose regimens is to limit overall antibiotic use to treat gonorrhoea despite high disease incidence.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~190 million gonorrhoea infections treated with a single, one-off antibiotic dose.
Urgency of AMR threat
N. gonorrhoeae has an extensive history of developing resistance to new agents and no single, reliable monotherapy to treat N. gonorrhoeae infection remains 282. Both the WHO and the CDC have expressed concerns about the future of gonorrhoea treatment: the WHO has listed N. gonorrhoeae as a ‘high priority’ for research and development of new treatments and the CDC has listed it as an ‘urgent’ AMR threat 6,7. The first antibiotic resistant strains of N. gonorrhoeae developed in the 1940s when sulphonamide-resistant strains emerged 289. By the end of the 1980s, resistance to penicillin was widespread and cephalosporins became the preferred treatment, but in 2011, a strain with high-level resistance to cephalosporin was reported 289. Resistance to several drug classes is now widespread, including macrolides, tetracyclines, and fluoroquinolones 289. Moreover, reports of extensively drug-resistant strains – resistant to both ceftriaxone and azithromycin – emerged in 2018 in the UK and Australia, leading to concerns that untreatable strains of N. gonorrhoeae could develop.
Scoring: Based on the above analysis, the urgency of AMR threat was characterised as high (score of 2 out of 2).
Pipeline robustness
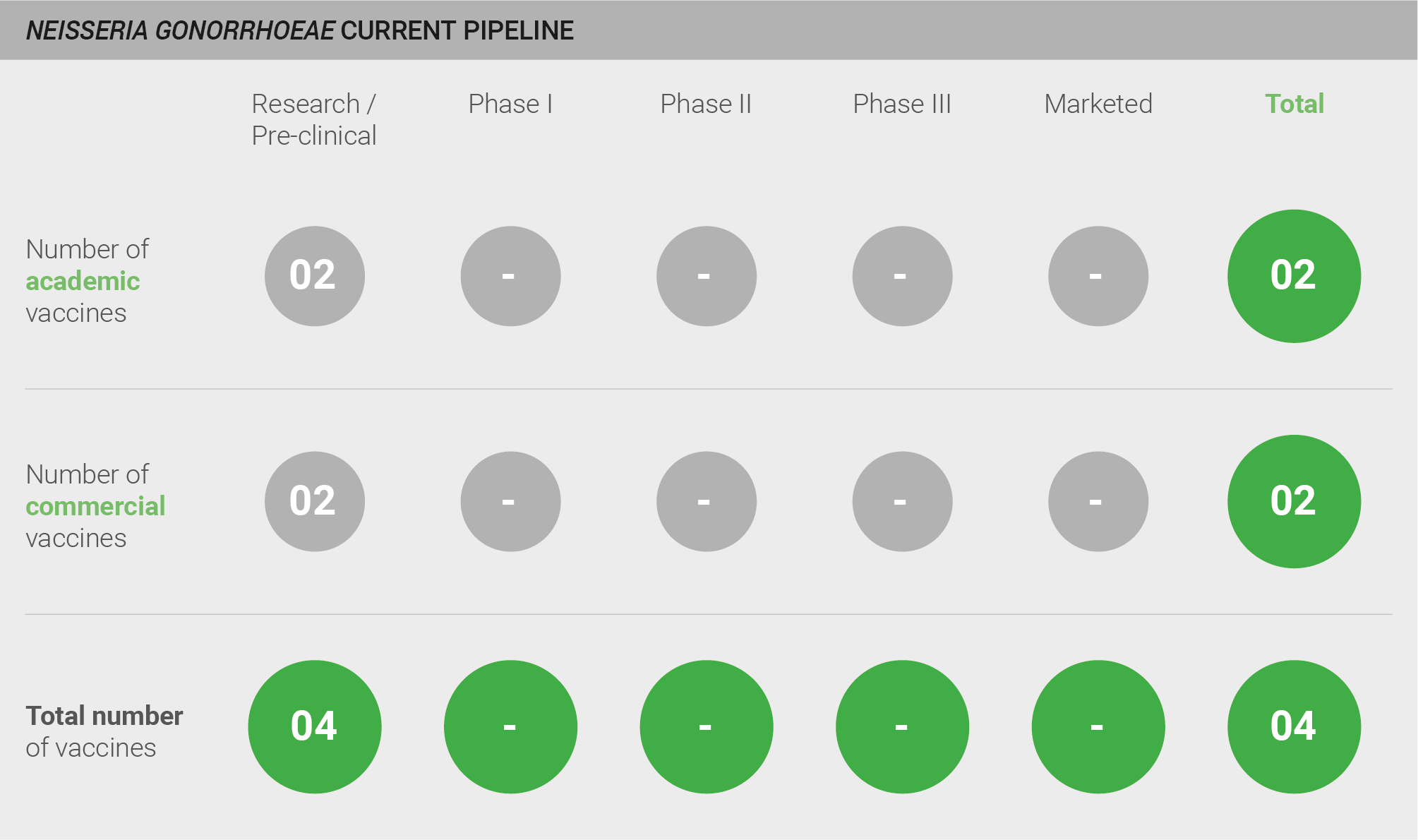
The pipeline for vaccines against N. gonorrhoeae is weak, with only four candidates in pre-clinical development. However, while acknowledging a lack of progress, experts are optimistic that a fully efficacious vaccine can be developed against N. gonorrhoeae – not least because of the result results of retrospective studies that show some evidence of cross-protection from the MeNZB N. meningitidis vaccine 285.
Scoring: Based on the above analysis, pipeline robustness was characterised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
The evidence for natural immunity against N. gonorrhoeae is not compelling. Repeated exposure to N. gonorrhoeae appears to be associated with a reduced risk of salpingitis (inflammation of the fallopian tubes) but does not appear to protect against uncomplicated infections 283. A study conducted in Nairobi suggested that women suffering repeated infections showed partial serovar-specific immunity against the prevalent circulating N. gonorrhoeae strain 290; however, this finding was not replicated in a study of less-exposed subjects in the United States 284. Experts’ opinions were consistent with this data; according to one expert “[we are] seeing some people getting gonorrhoea 12 times a year within a very small subset of the population” 28.
Several conserved targets have been identified, some of which have shown protection in pre-clinical mouse models. These include a 2C7 mimetic given with MAP1 adjuvant, OMV given with IL-12 and rrPorB-VRP (viral replication particle vector boosted with rrPorB + Ribi 700) 282. Additional candidates have shown the ability to induce antibodies with anti-gonococcal activity in mice, including TbpA, TbpB, AniA, and MtrE. No vaccine specifically targeting N. gonorrhoeae has been tested in humans to date.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
A large retrospective case-control study conducted in New Zealand demonstrated that the N. meningitidis MeNZB vaccine generated some cross-protection against N. gonorrhoeae infection, providing some encouraging support for development of a vaccine. This study examined the protective effect of the N. meningitidis MeNZB vaccine in 15-30 year-old participants born between 1984 and 1998 who were eligible for the MeNZB vaccine and had been diagnosed with gonorrhoea and/or chlamydia after attending one of 11 participating sexual health clinics in New Zealand. After controlling for ethnicity, deprivation, geographical area, and sex, the MeNZB vaccine demonstrated 31% efficacy in preventing N. gonorrhoeae infection 285. However, protection waned over time, indicating that higher titres may be needed 28. Experts suggest that higher titres could be achieved with multiple boosters, or that an alternative vaccine could achieve better efficacy.
Development of a vaccine against N. gonorrhoeae will require some obstacles to pre-clinical and clinical development to be addressed. As with most STIs, animal modelling in pre-clinical development is complicated by the human-specificity of the pathogen 282. A well-characterised female mouse model of lower genital tract infection is in place; however, this model is limited by the absence of several human-specific factors involved in adherence and invasion and the avoidance of complement-mediated killing of N. gonorrhoeae in humans. These factors include human transferrin and lactoferrin, soluble negative regulators of the complement cascade (factor H, C4b binding protein), receptors for gonococcal adhesins and invasins (i.e. CEACAMs), C3R integrin, CD46, and the pilus receptor. The development of transgenic mice expressing these absent host-factors could facilitate development of a N. gonorrhoeae vaccine, and in the absence of such a model, a combined approach incorporating challenge studies in normal mice and in vitro studies in human cells may provide insights into the efficacy of N. gonorrhoeae vaccines in humans.
Clinical development of a vaccine will also face some challenges. Experimental urethral infection of male subjects is possible due to the low risk of complication (for example, in kinetics studies, understanding the host response or virulence factors and similar studies). However, this might not reliably predict vaccine efficacy in women, or against complicated infection. Because of significant biological differences in gonorrhoea infection in men and women, men are likely to be a limited model for vaccine development in women 28.
The lack of established correlates of protection also presents a challenge for clinical development of a vaccine, as it limits researchers’ ability to understand what type of immune response a vaccine targeting N. gonorrhoeae needs to produce to provide protection against future infection. The observation of antibodies against gonococcal opacity proteins and absence of blocking antibodies in patients who show partial natural immunity against N. gonorrhoeae provides some insight into potential mechanisms of protection; however, the mechanisms by which N. gonorrhoeae manipulates host immune responses are not yet fully understood 282.
Trial infrastructure and design also present challenges for development of a vaccine for N. gonorrhoeae. Prospective efficacy trials would require participants to engage in unprotected sex, raising ethical questions about trial design. Furthermore, it may also be important to test a candidate vaccine in the context of a N. gonorrhoeae/chlamydia trachomatis (C. trachomatis) infection model, because C. trachomatis seems to create a more hospitable environment for N. gonorrhoeae. However, it is not clear how such a model could feasibly be implemented in a clinical trial.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
The most likely target population for vaccination against N. gonorrhoeae is adolescents and young adults prior to peak age of sexual activity. The most likely strategy for deployment of a vaccine would involve routine vaccination of this group. This could allow for a vaccination programme where the N. gonorrhoeae vaccine is delivered with the HPV vaccine or combined with the N. meningitidis vaccine to help drive uptake. If the duration of protection is limited, a second touchpoint would be required in later adolescence.
Development of a more targeted strategy is complicated by difficulty identifying and reaching a better-defined target population. Men who have sex with men (MSM), for example, are a high-risk group but may not be a large enough target population to achieve herd protection. Furthermore, MSM are unlikely to present for vaccination in countries where MSM are not widely accepted. Adolescents and young adults who have many sexual partners are also a high-risk group. However, this group is difficult to identify and targeting individuals with multiple partners may increase the stigma associated with the vaccine.
The WHO is supportive of vaccination due to high incidence and increasing treatment failures caused by antibiotic resistance 291–293. Similarly, the 2017 Chatham house report on vaccines supported a N. gonorrhoeae vaccine because of the poor antibiotic pipeline 294. However, the perception that N. gonorrhoeae is a low-risk pathogen with low mortality may generate some resistance to routine vaccination of all adolescents/young adults.
Scoring: Based on the above analysis, the expected policy stance was categorised as medium (score of 1 out of 2).
Likelihood of payer, government, or Gavi support
Vaccination against N. gonorrhoeae would likely benefit from payer support in high-income countries. Antibiotic-resistant N. gonorrhoeae infection incurs high costs – estimated at $500 million annually in the United States 294 – and an increase in drug-resistant strains would raise the risk of even higher costs. Governments in middle-income countries, however, may raise some concerns about cost-effectiveness, particularly the potentially lower cost-effectiveness of a routine vaccination strategy targeting all adolescents and young adults compared with a targeted strategy focusing on specific high-risk groups. Finally, Gavi is unlikely to support a N. gonorrhoeae vaccine under current prioritisation criteria because of the low mortality from gonorrhoea relative to other investment options. In the longer-term, Gavi support may be possible if their prioritisation criteria evolves to put stronger weight on AMR 28.
Scoring: Based on the above analysis, likelihood of payer, government, or Gavi support was categorised as medium (score of 1 out of 2).
Barriers to uptake
Some cultural and logistical considerations are likely to present obstacles to implementing a N. gonorrhoeae vaccination programme. The target population – adolescents and young adults – is generally a hard-to-reach group with sporadic contact with healthcare services. This could present challenges not only in initiating vaccination, but in ensuring multiple touchpoints if the duration of vaccine protection is limited. Uptake of a N. gonorrhoeae vaccine could be increased, however, if delivery in conjunction with a second vaccine is feasible. HPV and N. meningitidis present two potential candidates for a coordinated strategy. Delivery in combination with HPV could promote adoption of a N. gonorrhoeae vaccine in girls and young women; however, experts cite some challenges constraining adoption of the HPV vaccine that are also likely to affect a N. gonorrhoeae vaccine. Creating a combination vaccine against N. gonorrhoeae and N. meningitidis may better reduce the potential stigma associated with vaccines targeting STIs and benefit from a better-established healthcare touchpoint 28.
Experts agree that there is a stigma associated with vaccination against STIs that may cause resistance to widespread uptake. These experts explain that even for HPV – an STI with potential fatal outcomes – it is difficult to encourage widespread adoption among patients and their parents or caregivers. Because gonorrhoea is not fatal, healthcare providers may face even greater challenges in driving extensive adoption of a gonorrhoea vaccine. Some experts expect this stigma will be the most significant barrier to the implementation of a successful vaccination programme.
Experts also cite a clear need for healthcare provider education as part of any N. gonorrhoeae vaccination programme, even if the vaccine is administered alongside HPV, and particularly if the two vaccines cannot be administered on an identical schedule.
Scoring: Based on the above analysis, barriers to uptake were categorised as medium (score of 1 out of 2).
Commercial attractiveness
A N. gonorrhoeae vaccine is somewhat likely to be commercially attractive in high-income markets. Incidence is high in these markets; for example, the CDC estimates more than 800,000 cases annually in the US295. In addition, gonorrhoea causes high morbidity, with more than 500,000 years lived with disability. Finally, some sequelae such as infertility are expensive to treat, so payers may be able to reduce the cost burden of those sequelae through vaccination.
However, the potential barriers to uptake mean that companies may be reticent to pursue development, especially given the R&D challenges. The stigma related to STIs could result in poor uptake, similar to what has been observed to date for the HPV vaccine. Vaccine developers may also harbour concerns that the public will not perceive gonorrhoea as a significant enough risk to warrant vaccination.
Interest in developing a N. gonorrhoeae vaccine has increased in high-income countries in recent years, but there is not a clear consensus among experts regarding the commercial attractiveness of a vaccine. Some experts believe N. gonorrhoeae is a “commercially interesting target” 28, whilst others suggest that it may not be “big enough for pharma” 28.
Scoring: Based on the above analysis, commercial attractiveness was categorised as medium (score 1 out of 2).
Primary recommendation
Vaccine development could be accelerated by increasing the pace at which promising current and future pre-clinical candidates are moved from animal to human trials. N. gonorrhoeae infection is highly human-specific and, as such, success in pre-clinical trials may not translate to humans. Small human trials at an earlier stage are likely to provide more insight into the potential success of candidate vaccines than continued animal research.
Early clinical trials should build upon challenge trials already conducted in males. Specific areas of inquiry that merit consideration include clinical trials replacing N. meningitidis antigens with N. gonorrhoeae-specific antigens 28. Further, the findings of the New Zealand trial should be supported by additional studies detailing the protective effect of the N. meningitidis vaccine, including investigation of the duration of protective effect, the doses required for protection, and whether the vaccine protects against all strains of N. gonorrhoeae.
Despite the potential of a N. gonorrhoeae vaccine to reduce morbidity and AMR, the vaccine development pipeline is weak. Strong and sustained commitment is required to strengthen this pipeline, such that it better reflects the potential impact of vaccination.
Secondary recommendations
Focus on early stage R&D and pre-clinical research is still required. In particular, better understanding of the protective effect of the N. meningitidis vaccine against N. gonorrhoeae may allow investigators to explore substituting or altering N. meningitidis vaccine antigens to enhance protection against N. gonorrhoeae.
The potential for a combined vaccine with N. meningitidis should also be explored in greater detail. A combined N. meningitidis and N. gonorrhoeae vaccine would ease some of the uptake challenges, particularly around cultural acceptability, for the vaccine.