Salmonella Paratyphi
Salmonella Paratyphi (S. Paratyphi) is an infection with relatively low incidence that causes paratyphoid fever. S. Paratyphi causes ~25,000 deaths and ~10,000 years lived with disability per year. Whilst there is a vaccine for S. Typhi, vaccine development for S. Paratyphi lags behind due to less scientific and commercial interest. Nevertheless, development of a vaccine should technically be feasible. Due to low incidence, mortality and morbidity, uptake of a standalone vaccine is unlikely.
S. Paratyphi falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to incentivise the development of multi-pathogen/combination vaccines. The secondary recommendation is to accelerate clinical development.
Salmonella enterica serovar Paratyphi (S. Paratyphi) is a Gram-negative bacterium of the family Enterobacteriaceae. S. Paratyphi A and B cause enteric fever 342. S. Paratyphi A is the most common serovar 342, and referred to throughout this chapter if not specified otherwise. Experts report limited ongoing research into other serovars, with one commenting “I am not aware of much work on S. Paratyphi B and C, but these serovars are not a big problem” 28.
S. Paratyphi is spread by the faeco-oral route and rarely through direct human contact 334,335. Symptoms of infection with S. Paratyphi include high fever, headache, loss of appetite, vomiting, constipation or diarrhoea, and splenomegaly 343. Groups at highest risk of infection are individuals with increased susceptibility associated with gastric achlorhydria 344 and those with immunosuppressive illnesses such as AIDS 345.
Over three million cases of S. Paratyphi infection are reported per year 31, predominately in Asia 32. Unlike S. Typhi and invasive non-typhoidal Salmonella, S. Paratyphi is not common in sub-Saharan Africa 28,342.
Direct health impact
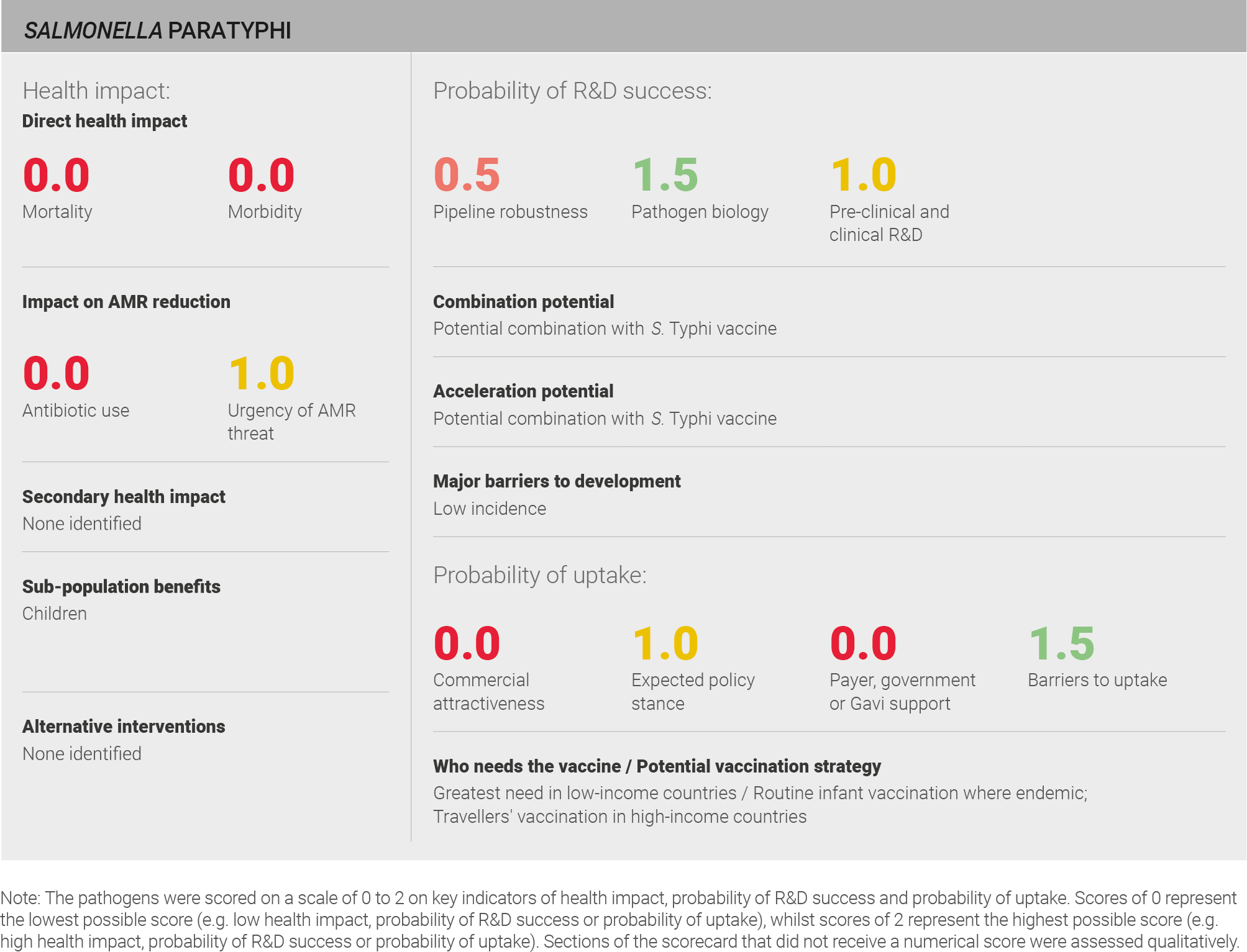
Data from the IHME 2016 estimates suggest low mortality (~25,000 deaths per year) and low morbidity (~10,000 years lived with disability per year) globally caused by S. Paratyphi infection 31. This source uses a defined methodology and is used in the global health community. The data can therefore be viewed with a reasonable level of confidence.
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
A vaccine against S. Paratyphi would provide benefits to infants and young children – the population at greatest risk for infection – and to patients in areas with elevated risk because of poor sanitation.
Antibiotic use
S. Paratyphi infection is primarily treated with fluoroquinolones, third-generation cephalosporins, and azithromycin. Carbapenems are reserved for suspected infection with extensively drug-resistant strains 346. Many experts consider fluoroquinolones to be the drug of choice for susceptible isolates and turn to azithromycin and cephalosporin when fluoroquinolones cannot be used.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score of 0 out of 2). This estimate is based on an annual incidence of ~ four million paratyphoid cases treated with a two week course of antibiotics.
Urgency of AMR threat
Salmonella spp. (the genus to which S. Paratyphi belongs) are listed as ‘high’ in the WHO priority list of research and development for new antibiotics. Multi-drug resistant strains resistant to ampicillin, trimethoprim-sulfamethoxazole and chloramphenicol are widespread, preventing these antibiotics from being used to treat S. Paratyphi infection 346. The presence of multi-drug resistant strains varies widely, from 10-80% in regions around the world 346, and full resistance to fluoroquinolones has also been reported. Most strains remain susceptible to azithromycin and ceftriaxone but resistance and extended-spectrum beta-lactamase producers have been reported 346.
Scoring: Based on the above analysis, urgency of AMR threat was categorised as medium (score of 1 out of 2).
Pipeline robustness
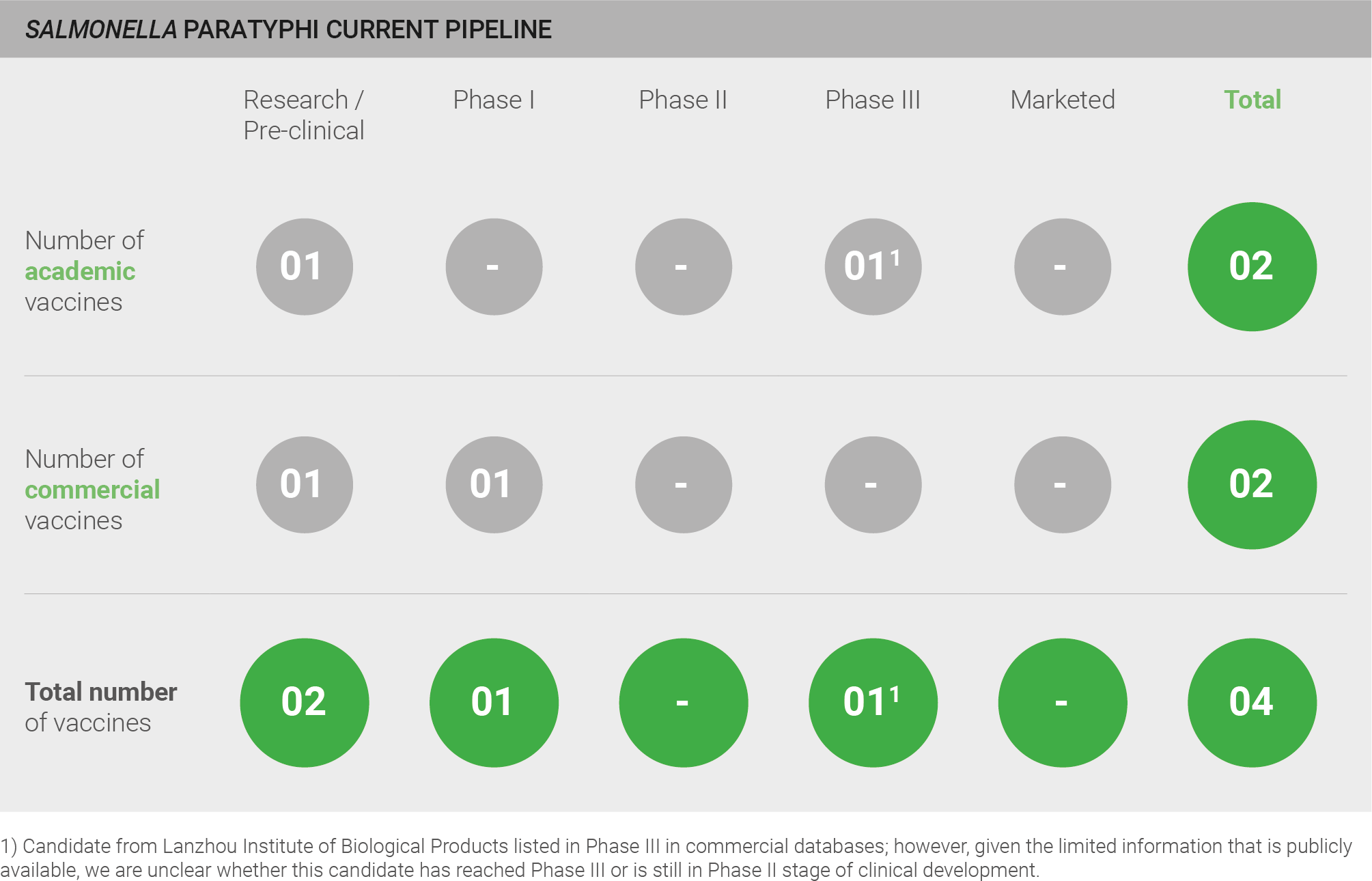
The pipeline for S. Paratyphi is not highly active: three vaccines against S. Paratyphi are currently in development. Two are in pre-clinical development, one is in Phase I. Another candidate from Lanzhou Institute of Biological Products is listed in Phase III in commercial databases; however, given the limited information that is publicly available, it is unclear whether this candidate has reached Phase III or is still in Phase II stage of clinical development.
Three out of the four vaccines captured by the analyses are bivalent vaccines comprising both a S. Paratyphi and a S. Typhi component to cover both typhoidal Salmonella strains342, reflecting the likely low uptake of a standalone S. Paratyphi vaccine. Experts are confident about the probability of R&D success for these vaccines in view of the recent success in developing the conjugated S. Typhi vaccine, which targets a pathogen with significant biological similarities to S. Paratyphi.
The scant activity in the pipeline does not reflect the feasibility of developing an efficacious vaccine against S. Paratyphi, but rather shows that commercial and academic researchers prioritised developing an S. Typhi vaccine, given the higher disease burden caused by typhoid fever.
Scoring: Based on the above analysis, pipeline robustness was categorised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
Natural and cross-strain immunity to S. Paratyphi appear to be limited; previous infection confers only partial protection against reinfection or disease severity 342.
The recently developed vaccine for typhoid fever – closely related to S. Paratyphi infection – targets the Vi antigen. As Paratyphi A and B do not express these antigens 347, the vaccine against S. Typhi does not induce cross-protection against these S. Paratyphi strains. Hence, a vaccine targeting paratyphoid fever will need to include a different set of antigens. Suitable options are O (polysaccharide antigens) and H (flagellar antigens) that are expressed by S. Paratyphi strains. The vaccine candidates in development therefore mostly target the O-specific polysaccharide (e.g. O:2 for S. Paratyphi A) conjugated to various different protein carriers 342 that enhance immunogenicity and convert the T-cell independent immune responses to a T-cell dependent response characterised by affinity maturation, subclass switching and induction of memory 348. Other examples for candidates in development are live attenuated pathogen 342 or vaccines based on Generalized Modules for Membrane Antigens (GMMA) 349.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly high (score of 1.5 out of 2).
Pre-clinical and clinical R&D
Models in mice have been the most utilised pre-clinical models. However, as S. Typhi and S. Paratyphi A are human host restricted and normally asymptomatic in mice, a lethal infection has to be produced in mice by suspending the bacteria in hog gastric mucin and then injecting the suspension intraperitoneally 350. This, however, does not accurately mirror the disease, which infects via the oral route and some researchers do not see existing models as clinically relevant 342. Results from other models 350 and larger animal models, such as non-human primates, may provide a more complete assessment of vaccine candidates and should be prioritised for vaccine candidates already evaluated in small animal models.
Clinical research involves several key challenges. There is an experimental human challenge model with S. Paratyphi A now established 351. However, correlates of protection have not been identified for S. Paratyphi A in humans. In vitro assays have quantified a positive correlation between serum antibody levels and in vitro bactericidal activity induced by either natural infection or immunisation, but this correlation is not yet adequately characterised to guide clinical trial design 342.
Experts also express concerns that although clinical trial infrastructure to run efficacy trials is in place in endemic regions, showing efficacy in field trials may be difficult and costly because of the low incidence of S. Paratyphi infection. This concern applies regardless of whether a vaccine is developed as a combination vaccine with a S. Typhi component or comprises antigens from other enteric diseases 28. The WHO published a guidance to develop a regulatory pathway for typhoid conjugate vaccines, but no such pathway has been developed for paratyphoid vaccines. The typhoid framework could serve as a surrogate until one is established 342; the S. Typhi vaccine was licensed on the basis of a Phase II study in an endemic setting that only demonstrated immunogenicity and safety. Efficacy was subsequently demonstrated in human challenge studies 352 and effectiveness studies are underway. A similar pathway to regulatory approval is anticipated for S. Paratyphi.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
A vaccine for S. Paratyphi would be particularly helpful for children in the endemic setting. A protective vaccine could therefore be administered to all children in endemic regions; travellers to endemic regions would also likely receive the vaccine.
Because S. Paratyphi has low incidence and low mortality 31, policymakers are unlikely to support a standalone vaccine, but as mentioned would prefer a combination vaccine with S. Typhi 28. Nevertheless, in a publication on paratyphoid fever vaccination recommendations prepared for the WHO Product Development for Vaccines Advisory Committee in 2014, vaccine development was thought to be a viable means for disease control as an adjunct to existing interventions 349.
Scoring: Based on the analysis described above, expected policy stance was characterised as medium (score of 1 out of 2).
Payer, government, or Gavi support
The low burden of disease in high and middle-income countries renders payer support unlikely. A combination vaccine is more likely to receive support as a travellers´vaccine.
Gavi has not taken any concrete steps towards designating a priority for vaccines against S. Paratyphi A. Gavi is unlikely to support a standalone vaccine but may support a combined enteric vaccine with S. Typhi 342.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as low (score of 0 out of 2).
Barriers to uptake
Few cultural and logistical barriers would prevent uptake of a S. Paratyphi vaccine. No new touchpoints would be required because the vaccine would likely be delivered as part of childhood vaccination programmes or offered to travellers. However, distribution could require additional infrastructure for supply chain and storage because the burden is predominantly in low-income countries.
No new clinical practices would need to be established for a S. Paratyphi vaccine. Childhood vaccines and travellers’ vaccines are routinely recommended by clinicians.
Scoring: Based on the above analysis, barriers to uptake was categorised as fairly low (score of 1.5 out of 2).
Commercial attractiveness
Commercial attractiveness of a standalone vaccine is low given the likely limited demand in high-income countries and middle-income countries, where the only market for a S. Paratyphi vaccine is for travellers. A combined S. Typhi/S. Paratyphi vaccine would be more commercially attractive than a standalone vaccine for S. Paratyphi; combinations with other enteric pathogens could also be attractive. As one expert states “It would be great to have a vaccine against ‘enteric disease’” 28.
Scoring: Based on the above analysis, commercial attractiveness was categorised as low (score of 0 out of 2).
Primary recommendation
The primary recommendation is to support research and development for a combination vaccine with S. Typhi. In the longer-term, it would be worthwhile to explore combination with other enteric vaccines given strong interest from policymakers in enteric vaccine combinations.
Secondary recommendation
The secondary recommendation is to accelerate clinical development. Key funders should be encouraged to support Phase III trials for promising combined S. Typhi/S. Paratyphi vaccine candidates. Gavi should be encouraged to provide an advanced market guarantee to pharmaceutical companies taking combined S. Typhi/S. Paratyphi candidates to Phase III trials. Finally, support for technical advances such as less expensive conjugation methods that enable cheaper vaccine production could help ensure uptake in low- and middle-income countries.