Escherichia coli (enteric)
Escherichia coli (E. coli) is a gut commensal that is part of the Enterobacteriaceae family, but is considered separately in this report because the higher burden of disease merits individual assessment. E. coli can be divided into multiple different subtypes. To facilitate a useful comparison two E. coli groupings were created – E. coli (enteric) representing those subtypes causing enteric infections (ETEC, EPEC, EIEC, EHEC and EAEC) and E. coli (urinary) representing UPEC infections.
Research and development efforts for enteric and urinary subtypes are clearly delineated by different target antigens and therefore vaccine design and primarily causes community-acquired infections in low- and middle-income countries. Enteric E. coli causes >60,000 deaths and almost 400,000 years lived with disability annually. Whilst resistance remains less common than for other Enterobacteriaceae, resistance to first-line agents and reports of extensively resistant strains are increasing. Dukoral, a cholera vaccine, has demonstrated a partial short-term cross-protective effect and vaccine candidates based on antigens that target most enteric E. coli strains are in clinical development. There is policy support for a standalone vaccine but funder support is likely dependent on further study regarding burden of disease.
Enteric E. coli falls into a cluster of pathogens for which bringing a vaccine to market is the priority. The primary recommendation is to accelerate clinical development. The secondary recommendations are to better understanding of pathogen burden, epidemiology and transmission, to incentivise the development of combined enteric vaccines, and to support pre-clinical research.
E. coli is a Gram-negative commensal that predominantly causes community-acquired infections, but can also cause hospital-acquired infections. Although E. coli is part of the Enterobacteriaceae family, it has been considered separately in this assessment because of its high incidence relative to other members of the Enterobacteriaceae family.
E. coli is part of the normal gut flora, with pathogenesis caused by several strains 131. Most presentations from enteric E. coli are caused by Enterotoxigenic E. coli (ETEC). Other disease-causing pathotypes include Enteropathogenic E. coli (EPEC), Enterohaemorrhagic E. coli (EHEC), and Enteroaggregative E. coli (EAEC) 131. The analysis in this chapter pertains to ETEC and EPEC. Where aggregated information on all pathotypes is included for context, it is clearly noted as such.
Enteric E. coli is transmitted through the faeco-oral route, primarily through contaminated food and water 132. Each type of enteric E. coli can have distinct clinical features: for ETEC, these include malaise, anorexia, and abdominal cramps followed by the sudden onset of watery diarrhoea 131; for EPEC, these include loose, watery stools, vomiting, and low grade fever 131. Enteric E. coli affects young children with high incidence before the age of three 133. Enteric E. coli infection is concentrated in low- and middle-income countries, affecting most of Asia, the Middle East, Africa, Mexico, and Central and South America 134.
Direct health impact
Global data regarding the disease burden associated with ETEC and EPEC is available from IHME. Data from these sources suggest a relatively low mortality compared to other pathogens on the WHO priority pathogen list, at ~60,000 deaths annually. Over 50,000 of these driven by ETEC 31. Morbidity is reported at 400,000 years lived with disability annually, also mostly driven by ETEC 18.
Data on mortality and morbidity was taken from the IHME 2016 estimates. The IHME has a defined methodology and their data is accepted in the global health community.
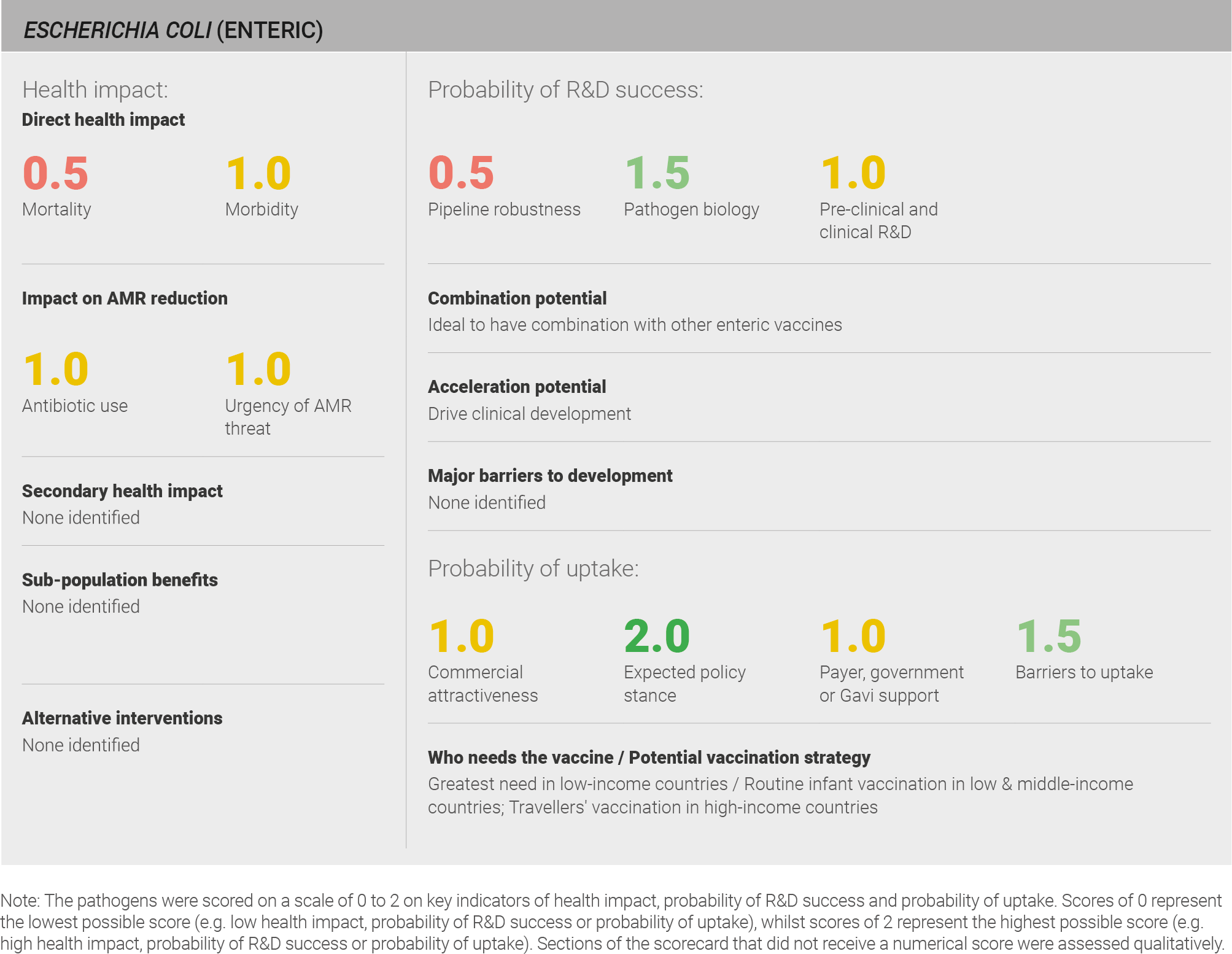
Scoring: Based on the above analysis, mortality was categorised as low (score of 0.5 out of 2) and morbidity was categorised as medium (score of 1 out of 2).
Secondary health impact
There is limited and conflicting data regarding the secondary health impact of enteric E. coli. Some research suggests an impact of diarrhoeal disease on growth trajectories for children, especially those with multiple diarrhoeal episodes 67,68. However, it is possible that these children return to normal growth and ultimately achieve normal milestones 69.
Sub-population benefits
Young children would benefit most from a vaccine, as enteric E. coli infection primarily affects young children with high incidence before the age of three 133.
Antibiotic use
Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles. A common regimen is a three-day oral course of a fluoroquinolone antibiotic 135.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~320 million enteric E. coli cases treated with a three day course of antibiotics.
Urgency of AMR threat
While there are strong concerns from international health bodies regarding threat of antibiotic resistance from Enterobacteriaceae, literature review suggests that there is less concern regarding resistant enteric E. coli strains than resistant strains in other members of the Enterobacteriaceae family.
Both the WHO and CDC have expressed strong concern about antibiotic treatments for Enterobacteriaceae (E. coli is not scored separately). The WHO has listed the Enterobacteriaceae group as a ‘critical’ priority for R&D regarding new antibiotics 31. The CDC has listed CRE (carbapenem-resistant Enterobacteriaceae) as an ‘urgent’ threat in its list of greatest threats from AMR and has listed extended spectrum Enterobacteriaceae as a ‘serious’ threat 7.
International concerns are partly driven by reports of E. coli strains resistant to polymyxin antibiotics – a last line therapy 136. Whilst there are reports of polymyxin resistance in enteric E. coli isolates 137,138, clinical practice remains, where indicated, to treat with fluoroquinolones or azithromycin. Despite growing resistance rates these therapies are still useful treatment options in many settings 139.
Scoring: Based on the above analysis, the urgency of AMR threat for Enteric E. coli was categorised as medium (score of 1 out of 2).
Pipeline robustness
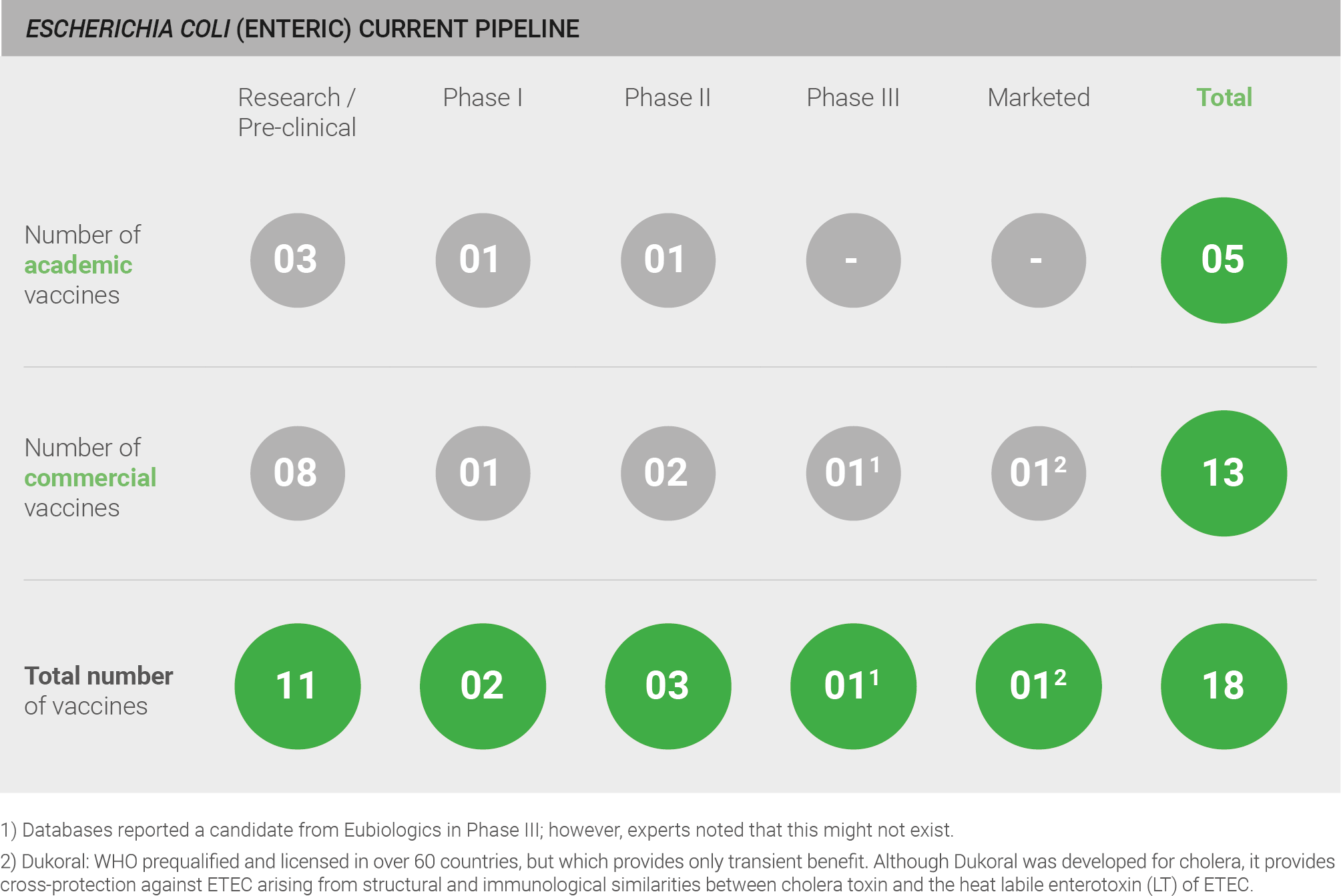
There is a moderate pipeline for a vaccine targeting enteric E. coli, comprising a total of 18 candidates; however, all are in early stages of development, with 11 pre-clinical candidates and five clinical candidates 40–42. All candidates currently in development target ETEC.
One candidate was reported to be in Phase III clinical trials; however, experts noted that this candidate likely does not exist.
There is also one marketed vaccine – Dukoral – that is WHO prequalified and licensed in over 60 countries, but which provides only transient benefit. Although Dukoral was developed for cholera, it provides cross-protection against ETEC arising from structural and immunological similarities between cholera toxin and the heat labile enterotoxin (LT) of ETEC. The protective effect of Dukoral is moderate, with efficacy estimated at 40-70% and only estimated to reduce up to 7% of cases of travellers’ diarrhoea from all causes 140. Additionally, this vaccine has only a short duration of protection – estimated at approximately three months 130.
Scoring: Based on the above analysis, pipeline robustness was categorised as medium (score of 1 out of 2).
Current pipeline
Pathogen biology
Field studies and human challenge studies indicate that protective immunity to ETEC does develop 141. Age-specific attack rates for symptomatic ETEC infection decline after three years of age; also, in human challenge studies, subjects who recovered from ETEC diarrhoea were protected against disease upon re-challenge 141. However, ETEC strains are antigenically highly diverse, meaning that there is little cross-strain immunity 28,141.
Two key potential vaccine targets have been identified that will likely cover 70-80% of strains: the LT toxoid and colonisation factor antigens (CFAs)28,142. All three clinical candidates discussed in expert interviews are aimed at these targets. CFAs are good candidates since they together cover 50% of clinical isolates 141. Although over 25 CFAs that have been identified, there are four antigens which are most frequently encountered, and which together are typically used in vaccine candidates 143. The two ETEC enterotoxins, heat stable (ST) and LT, also represent potential vaccine targets. LT is structurally, functionally, and immunologically related to the cholera toxin, hence the cross-protection of Dukoral. LT is easier to produce in a toxoid from, enabling immunogenicity without toxicity. It has been demonstrated as suitable for development through in vitro and in vivo studies 141.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly high (score of 1.5 out of 2).
Pre-clinical and clinical R&D
Pre-clinical research to develop an ETEC vaccine is limited by less than ideal animal models for ETEC and lack of correlates for protection. Historically, animal models of disease have not naturally developed diarrhoea after ETEC infection 141. A new mouse model with zinc deficiency shows promise, displaying growth impairment, watery diarrhoea, and intestinal inflammation after ETEC infection 72. Furthermore, rabbits, pigs and non-human primates could be used as they develop diarrhoea with infection. In summary, while current animal models are imperfect, there is promising work that may lead to better models.
Human challenge models have made clinical research easier than pre-clinical work 141. While there has been longstanding and successful use of challenge models, there are three ways to improve their use. First, there is scope for improvement through with increased fasting time prior to challenge, which enables a lower dose that better mimics natural field exposure. Second, there is also scope to expand use of challenge models – moving beyond the strains where they have been employed. Third, establishing correlates of protection and functional assays predicting immunity would enable shorter, more efficient trials.
Trial infrastructure is likely be conducive for vaccine development, since trials for other diarrhoeal diseases such as cholera have been successful in similar settings. Vaccine candidates avoid targeting antigens expressed by commensal E. coli, as toxoids are only present with pathogenic E. coli, and the presence of fimbriae are correlated with pathogenicity 144 . This means that trials may be able to avoid the regulatory burden and additional expense associated with monitoring impact on commensal E. coli. Route to licensure should be relatively straightforward given ETEC vaccine candidates exploit longstanding vaccine technology, such as the use of protein vaccines.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
A vaccination strategy for ETEC would include routine vaccination in the first year of life in low- and middle-income countries, and vaccination offered for travellers from high-income countries traveling to higher-risk areas.
Vaccination is likely to be supported by policy bodies. This is due to the high incidence of enteric E. coli 18,74 and the fact that the WHO is assessing E. coli vaccine candidates for accelerated clinical development 145. A document prepared at the request of WHO PDVAC states “There are currently no licensed vaccines for ETEC, but studies indicate high public health impact, cost-effectiveness, and feasibility of immune protection through vaccination. ETEC vaccine development remains a World Health Organization priority” 141. However, fluctuations in IHME estimates of mortality for ETEC have prompted desire for further data on disease burden from the policymaking community.
Scoring: Based on the above analysis, expected policy stance was categorised as high (score of 2 out of 2).
Payer, government, or Gavi support
The low mortality burden in high-income countries is likely to lead to a lack of payer support for an ETEC vaccine except as a travel vaccine. In middle-income countries, ETEC vaccination support will likely depend on the price per dose, given that particular subpopulations would benefit greatly from the vaccine. In low-income countries, the route to market would likely be through Gavi. While an analysis by the Vaccine Alliance suggested that there would be a favourable cost-effectiveness ratio of $65.00/DALY 141, the flux in mortality estimates has made cost-effectiveness more difficult to justify 141.
Scoring: Based on the above analysis, payer, government or Gavi support was categorised as medium (score of 1 out of 2).
Barriers to uptake
The logistical barriers to implementing a vaccination programme for enteric E. coli are relatively low; it would not likely require a new healthcare touchpoint and would likely be incorporated into the childhood vaccination schedule. Clinical practices would also present few barriers; as routine vaccination in infancy, an enteric E. coli vaccine would use a familiar route of delivery and no change in clinician behaviours would be required.
Scoring: Based on the above analysis, barriers to uptake were categorised as fairly low (Score 1.5 out of 2).
Commercial attractiveness
A 2011 PATH analysis suggests that a low-cost ETEC vaccine could have an estimated annual revenue potential of more than $600 million at maturity, with greatest uptake in high-income (travellers’ vaccine) and mid-income countries 146. An ETEC vaccine could compete with Dukoral as a travellers’ vaccine; however, it is uncertain Gavi will support an ETEC vaccine without further evidence on disease burden.
Scoring: Based on the above analysis, commercial attractiveness was categorised as medium (score of 1 out of 2).
Primary recommendation
The primary recommendation is to accelerate clinical development of a vaccine. Increasing funding for later-stage clinical trials would likely accelerate clinical development. Key funders of enteric disease research in commercial and non-commercial spheres should be encouraged to invest in larger clinical trials for early-stage clinical research candidates, especially given that there are two Phase I candidates. Opportunities for funders to strategically coordinate efforts so they are able to pool resources and fund later-stage trials for enteric E. coli would also help accelerate clinical development.
Clinical development should also be accelerated through a focus on regulatory facilitation. A vaccine for Salmonella Typhi has recently been prequalified by the WHO based on evidence from human challenge models 147,148. Similar options could be explored for enteric E. coli. Human challenge models for the ETEC have been used to measure the efficacy of vaccine candidates, so use of these models could be increased for later-stage trials 141.
Secondary recommendations
One secondary recommendation is to gain a better understanding of pathogen epidemiology. IHME figures for enteric E. coli disease burden in 2015 have decreased compared to 2010 31. The apparent decrease in disease burden suggested by IHME has prompted some funders to leave the field 149, which increases the difficulty of implementing later stage trials. There is expert concern that the IHME numbers may underestimate the burden of disease 28,150. In 2015, IHME moved towards using molecular methods (quantitative PCR) in burden of disease estimates. Since these methods have greater sensitivity than stool culture, there has been an increase in detection of ETEC as well as other pathogens; however, the increase in ETEC detection has not been as pronounced compared to other pathogens 150. Not all ETEC serotypes produce the ST toxin that is detected in the assay 142. There have also been changes in modelling methodology 142,150. Incorporation of data from two large observational studies, MAL-ED and GEMS, into global burden of disease estimates is in process and will provide a more comprehensive picture of disease burden 150.
Further studies would develop the understanding of disease burden in two distinct directions 141,150. First, broader datasets would help to reduce extrapolations over age ranges and imputation; second, smaller, more detailed studies would enable maximally accurate diagnostics to be used for E. coli and other infections, maximising the diagnostic yield from incident symptomatic cases, minimising misdiagnosis, and influencing how data from broader studies can be modelled.
Another secondary recommendation is to support development of combined enteric vaccines. There is a stated interest from policy bodies and funders to explore combination vaccines. There are currently combined Shigella-ETEC vaccines in pre-clinical and Phase I clinical development 42.
Last, expanding pre-clinical research, including selection of animal models, is recommended. Further development of non-mouse, non-pig animal models such as rabbits or primates could provide additional data or models with improved predictive capacity for clinical development. Efforts to increase pre-clinical research should also promote development of platforms that enable manufacturing of inexpensive multivalent vaccines. Bioconjugation, for example, involves the binding of one or more antigens from a pathogen to a Toll-like receptor ligand, which enhances the immune response 149. Multiple components can be combined in this process, resulting in an immune response to more than one pathogen or to several elements of a single pathogen 151. This approach has been used in urinary E. coli trials, and a similar approach would likely be possible in enteric E. coli 152. Given the commensal nature of the pathogen, pre-clinical research should also seek to better understand the potential effect of vaccines on gastrointestinal flora.