Enterococcus faecium
Enterococcus faecium (E. faecium) causes hospital-acquired infections and has low incidence, morbidity and mortality. It most commonly presents as a urinary tract infection or endocarditis. A potential vaccination strategy and target population have yet to be identified. AMR is of intermediate concern as resistance to last-line therapies has not been reported. There are no vaccine candidates in pre-clinical or clinical development. A vaccine against E. faecium is unlikely to be cost-effective given the high cost of developing vaccines and the low incidence of infection.
E. faecium falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to explore alternative treatment and prevention strategies. The secondary recommendation is to better understand the burden, epidemiology and transmission of E. faecium infection.
E. faecium is a Gram-positive, commensal bacterium that causes hospital-acquired infections. E. faecium is commonly found in the gastrointestinal tract; it can also rarely be present in the oral cavity or the genitourinary tract 116. Colonization with E. faecium can occur through person-to-person contact or exposure to contaminated objects 116.
The most common manifestations of infection are urinary tract infections (UTIs); however, E. faecium can cause a range of other infections including endocarditis and meningitis. Symptoms vary depending on the infection site, but can include the following:
- UTI: Frequency; urgency, dysuria, discolouration of urine (urine can appear cloudy, red, pink or cola-coloured) and pelvic pain in women
- Endocarditis: fatigue, malaise, night sweats, shortness of breath, new or changed heart murmur
- Meningitis: headache, neck stiffness, photophobia, nausea and vomiting, confusion, drowsiness, seizures
E. faecium infection has a global distribution 117 but is typically found only in specific at-risk groups. Groups at greatest risk for infection with E. faecium include immunocompromised individuals, particularly patients admitted to intensive care units 116, and patients with other concomitant conditions 28. As one expert explains, “Enterococcus is found only in multimorbid patients, in addition to other underlying diseases”.
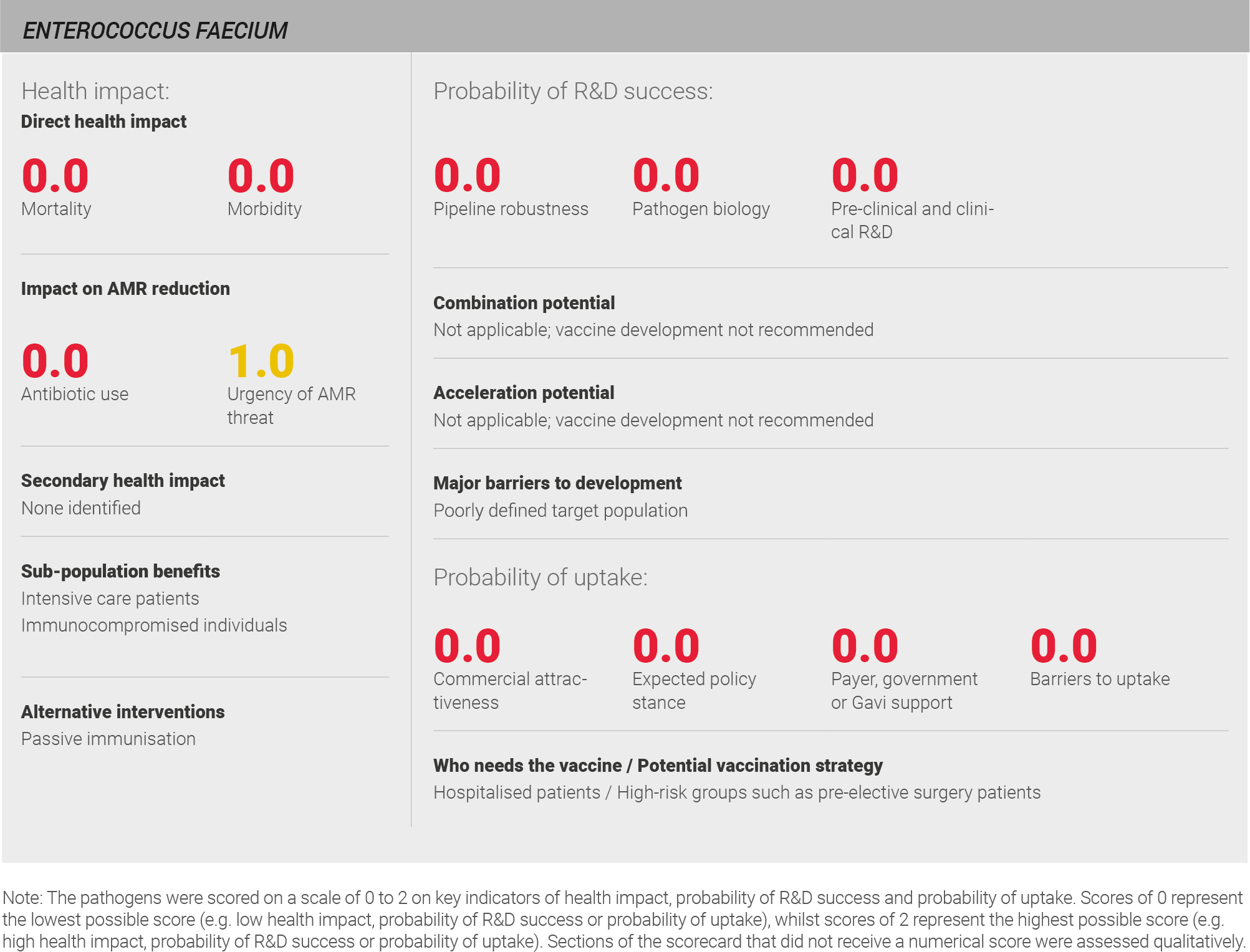
Direct health impact
Global data on the direct disease burden of E. faecium infections is not available from either the WHO or the IHME. A review of the literature suggests that E. faecium is responsible for approximately 3% of UTIs and 4% of endocarditis cases 82,114,115. Based on the limited availability of data, it is challenging to assess the global burden of E. faecium infection with confidence. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2). Morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
A vaccine for E. faecium would benefit immunocompromised patients, particularly in intensive care settings.
Antibiotic use
The course of treatment for E. faecium infection varies depending on the specific infection. A typical treatment course for a UTI is a 7-day course of antibiotics 118. For infective endocarditis, treatment typically employs multiple agents in combination for approximately six weeks 119. The low incidence of E. faecium infection drives low overall antibiotic use.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score 0 out of 2). This estimate is based on an annual incidence of ~50,000 endocarditis cases treated with a six week course of antibiotics, and ~12 million UTIs seven day week course of antibiotics
Urgency of AMR threat
Both the WHO and CDC have expressed concern about the future of E. faecium treatment. The WHO has listed it as a ‘high’ priority for research and development of new antibiotics 6 and the CDC has listed it as a ‘serious’ AMR threat 7. Resistance to penicillin and aminoglycosides has been widely reported 120 and some strains are also resistant to vancomycin (vancomycin resistant enterococcus [VRE]) 120. Rates of VRE are highest in North America 116. The United States Food and Drug Administration (FDA) has approved the use of linezolid for the treatment of VRE. Other last-line options are also available, including daptomycin 121.
Scoring: Based on the above analysis, the urgency of AMR threat was characterised as medium (score of 1 out of 2).
Pipeline robustness
There is currently no pipeline for vaccines against E. faecium; neither commercial nor academic vaccine development programmes are underway.
Scoring: Based on the above analysis, the pipeline was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
The biology of E. faecium is presently not well characterised and little is known about natural or cross-strain immunity following the commensal-to-pathogen switch 122. Vaccine targets are also not understood in detail at this time; structural characterisation of E. faecium has been limited to the identification of teichoic acids, including lipoteichoic acid and a wall teichoic acid, and definitive structures of high molecular weight polysaccharides – for example, Pfl1-4 – have only been described recently 123.
Scoring: Based on the above analysis, pathogen biology was categorised as low (score of 0 out of 2).
Pre-clinical and clinical R&D
No pre-clinical or clinical trials of an E. faecium vaccine have been conducted to date. Mouse models and opsonophagocytic assays are currently being used to study the pathogen, but the clinical relevance of these models has not yet been determined as no vaccine candidates have reached clinical development.
The implementation of clinical programmes is also limited by the lack of knowledge about natural immunity following the commensal-to-pathogen switch and the lack of known correlates of protection that could simplify outcome measures in clinical studies. Finally, the target population for clinical studies is currently unclear.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as low (score of 0 out of 2).
Expected policy stance
It is not clear at this time how a vaccination programme for E. faecium infections would be implemented as both the target population and vaccination strategy are presently unclear. Because infection with E. faecium is limited to patients with concomitant health conditions or compromised immune systems, the healthy population is unlikely to benefit from vaccination. As one expert notes, “You are not going to develop a vaccine that you would give to the healthy population” 28. Immunocompromised patients are most likely to benefit, but they are a complex population to effectively vaccinate. This is because whilst live attenuated vaccines are potentially pathogenic in this population, these patients may be unable to mount an adequate immune response to subunit and inactivated vaccines to ensure protection 124.
Expert interviews reflected a lack of enthusiasm to prioritise support for vaccines against E. faecium. The pathogen is not well-known to policy makers and experts cited the small number of affected patients as a barrier to policy maker support, explaining “vaccines here wouldn’t make a lot of sense because it wouldn’t have a big impact” 28.
Scoring: Based on the above analysis, the expected policy stance was categorised as low (score of 0 out of 2).
Payer, government, or Gavi support
No expert interviews or other research indicated that E. faecium infection is a high priority for payers or governments in high-income or middle-income countries. Gavi support is also unlikely; mortality in Gavi countries is not known and is likely to be relatively low.
Scoring: Based on the above analysis, likelihood of payer, government, or Gavi support was characterised as low (score of 0 out of 2).
Barriers to uptake
The primary barrier to uptake of a vaccine for E. faecium is the difficulty in identifying a target population; as noted previously, immunocompromised patients are most likely to benefit, but formulating a strategy to vaccinate this population would likely be complex. Because E. faecium is not a well-known pathogen, extensive healthcare provider and patient education would be necessary. Implementation of a vaccination programme for E. faecium would also require changes to some clinician behaviours, and clinicians would need to understand and support incorporation of vaccination into patient pathways.
Scoring: Based on the above analysis, barriers to uptake were categorised as high (score of 0 out of 2).
Commercial attractiveness
Disease caused by E. faecium is low incidence, there is low likelihood of Gavi support and the target population is not clearly defined, therefore the commercial attractiveness of the pathogen is low.
Scoring: Based on the above analysis described above, commercial attractiveness was categorised as low (score of 0 out of 2).
Primary recommendation
The comparatively low incidence, morbidity and mortality associated with E. faecium infection, coupled with the costs of vaccine development, mean that E. faecium is not a strong candidate for vaccine development. The primary recommendation is to explore alternative treatment and prevention strategies, such as passive immunisation strategies and the prevention of biofilm formation on urinary catheters. Passive immunisation strategies are time-consuming and expensive to develop and require sufficient understanding of the pathogen biology, which is currently a hurdle for E. faecium.
Secondary recommendation
The disease burden of E. faecium infection is currently not well-characterised, and additional epidemiological studies would likely provide a better understanding of disease burden, especially in low-income countries where information is currently scarce. This information will be helpful for the understanding of the pathogen but will be unlikely to change the recommendations on vaccine development.