Campylobacter spp
Campylobacter causes community-acquired infections that present as acute gastrointestinal illness and are often transmitted through undercooked meat, especially poultry. It causes significant morbidity (~290,000 years lived with disability annually) but limited mortality (~75,000 fatal cases annually). The current understanding of pathogenesis, protective epitopes and antigenic diversity is incomplete, providing researchers with few starting points for vaccine development. The limited mortality burden and relatively minor symptoms associated with Campylobacter makes uptake challenging. A vaccine is unlikely to be deemed cost-effective and would therefore most likely be limited to use as a travellers’ vaccination and amongst military personnel in high-income countries.
Campylobacter falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to better understand the burden, epidemiology, and transmission. The secondary recommendations are to explore alternative treatments or prevention strategies and to incentivise the development of combination vaccines for enteric diseases.
Campylobacter is a Gram-negative bacterium that causes community-acquired infections. Campylobacter is not a normal part of the human gut microbiome 60. Campylobacter infection typically presents as gastroenteritis with diarrhoea, fever, abdominal pain, and vomiting 60. However, the disease is usually mild and self-limiting. As such, maintenance of proper hydration (including electrolyte correction) should be the focus of therapy. Antibiotics are not needed for most cases of Campylobacter-associated gastroenteritis but are often prescribed 60.
Typical routes of transmission vary somewhat by region. In high-income countries, Campylobacter is transmitted through undercooked meat, especially poultry 60. In low-income countries, however, many aspects of transmission are not well-characterised. It is not clear from what sources, how, and where individuals contract infections. It is also not known what role, if any, domestic animals play in infection, whether faecal contamination in the environment can cause infection, and how long Campylobacter can survive in the environment outside of a host. The role of host undernourishment in susceptibility to infection is also not well understood. Finally, the relevance of transmission to neonates and younger children within households is not yet understood. The Bill and Melinda Gates foundation has recently started an initiative to elucidate the transmission dynamics of Campylobacter in low- and middle-income countries 61.
Campylobacter is globally distributed, with higher incidence of infection observed in the WHO African, South East Asian, and Western Pacific regions 18. Incidence of Campylobacter is higher in children than adults, particularly in Africa, South East Asia, and the Middle East 62. Immunocompromised populations, including patients with AIDS, are at risk of disseminated infection 63.
Direct health impact
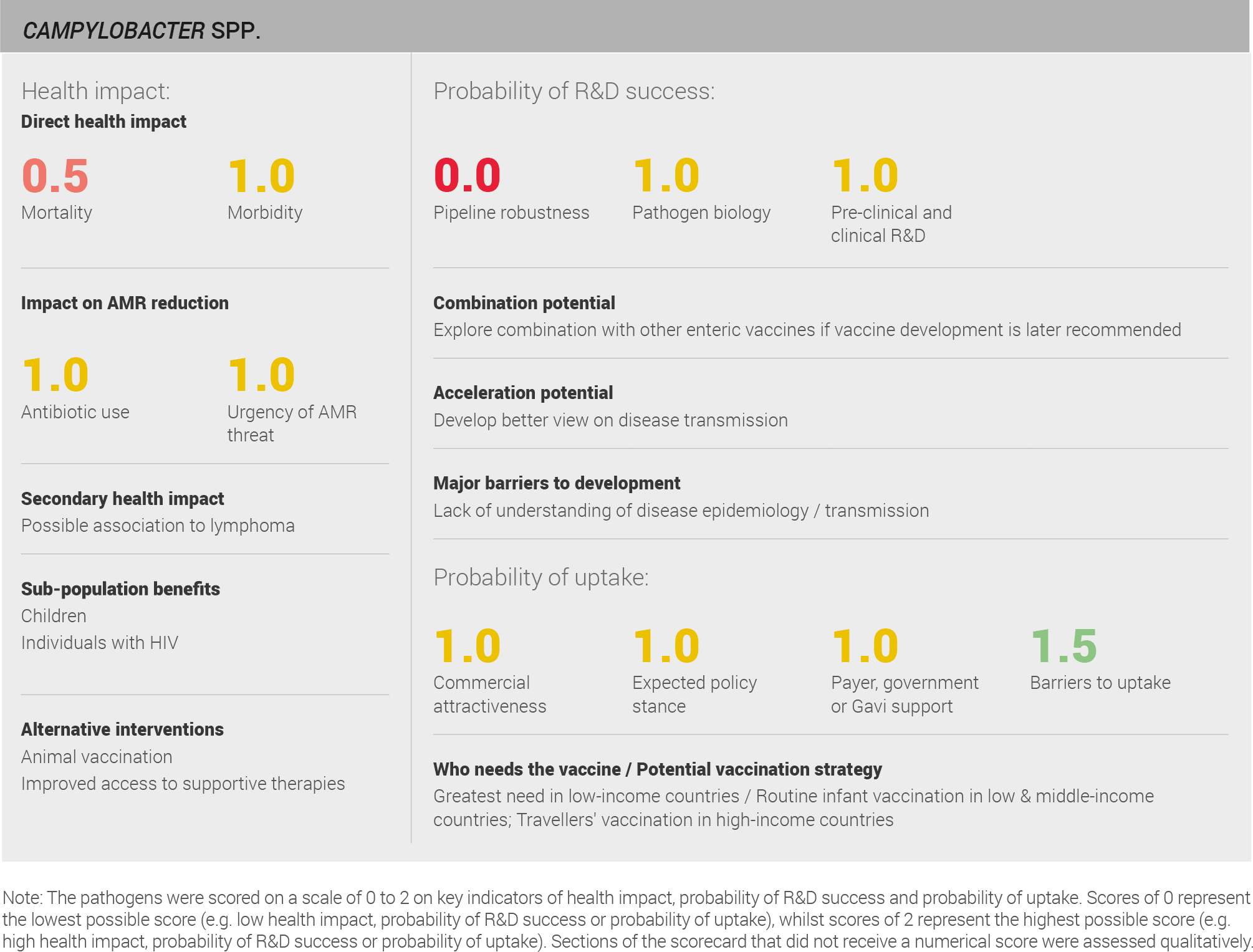
Data on morbidity and mortality are available from the IHME 2016 estimates 64. This source uses a defined methodology and is used in the global health community. The data can therefore be viewed with a reasonable level of confidence. The IHME estimates mortality from Campylobacter infection at approximately 75,000 cases per year and morbidity approaching 290,000 years lived with disability annually. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was categorised as fairly low (score of 0.5 out of 2) and morbidity was categorised as medium (score of 1 out of 2).
Secondary health impact
Evidence from lymphoma patients showing improvement in their cancer after antibiotic treatment, as well as biopsy specimens from cancer patients suggest a possible link between Campylobacter and lymphoma 65,66. Some research suggests an impact of diarrhoeal disease on growth trajectories for children, especially amongst children with multiple diarrhoeal episodes 67,68. However, it is possible that these children experience catch-up growth and return to normal growth trajectories 69.
Sub-population benefits
Children in regions with high incidence of Campylobacter infection – particularly Africa, South East Asia, and the Middle East 70 would benefit from a vaccine since children have a higher rate of Campylobacter infection. Immunocompromised populations such as patients with AIDS who are at risk of disseminated disease would also benefit.
Antibiotic use
Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles. Regimens vary in length but a typical course is three days of a macrolide or fluoroquinolone antibiotic 60.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~90 million Campylobacter infections treated with a three day course of antibiotics
Urgency of AMR threat
Both the WHO and CDC have expressed concern about antibiotic treatments for Campylobacter infections. The WHO has listed Campylobacter as a high priority for R&D regarding new antibiotics 31 and the CDC has listed Campylobacter as a “serious” threat in its list of greatest threats from AMR 7.
Campylobacter is inherently resistant to trimethoprim and beta lactams 60. Macrolides are still usually effective even in geographies where resistant strains are more common 60. Fluoroquinolone resistance exceeds 80% in South East Asia and is on the rise globally 60. However, alternative antibiotic treatments remain effective, including carbapenems, and aminoglycosides60. Campylobacter is typically also sensitive to clindamycin, tetracyclines, and chloramphenicol, although there is no data on the clinical efficacy of these antibiotics 60.
Scoring: Based on the analysis described above, the urgency of AMR threat was categorised as medium (score of 1 out of 2).
Pipeline robustness
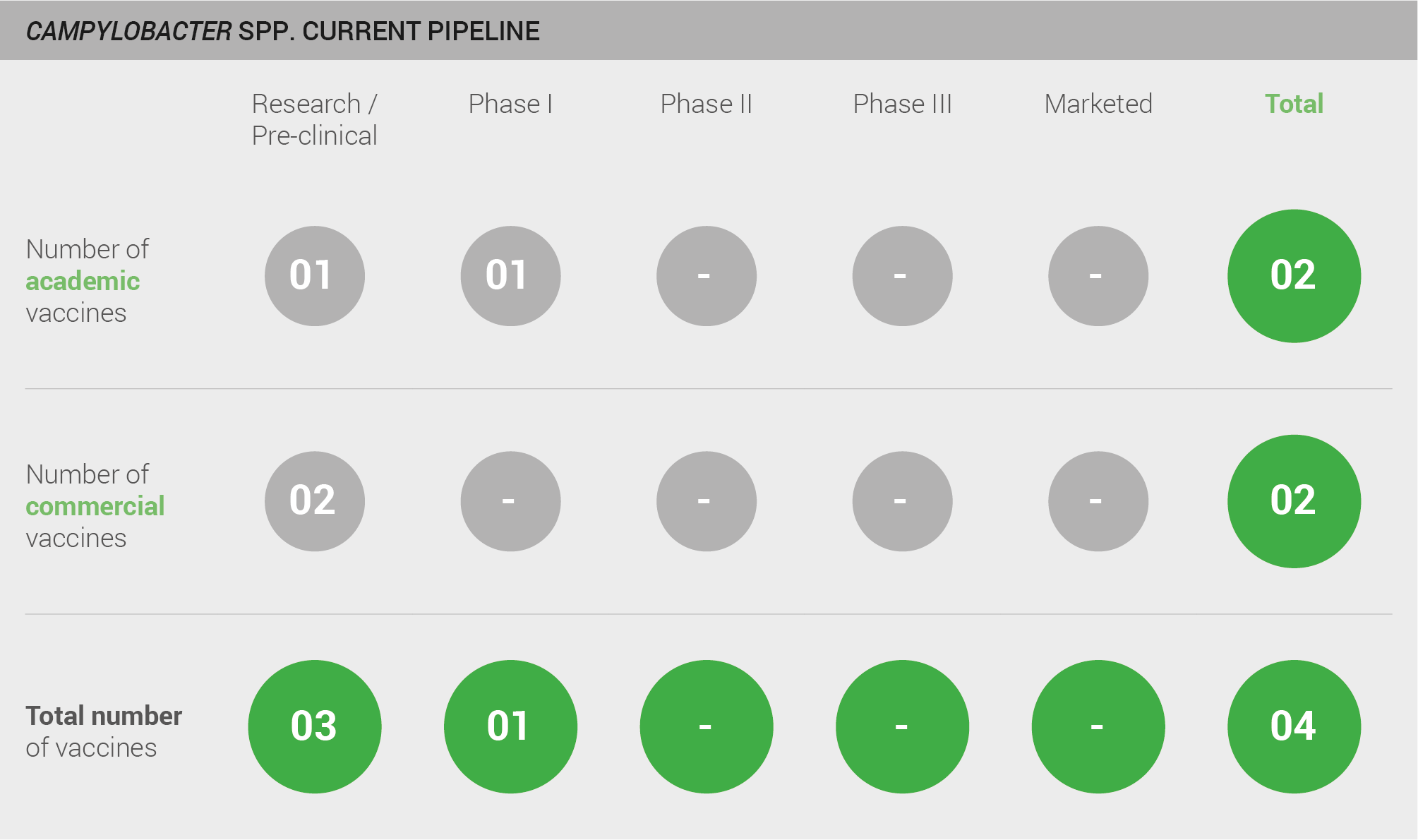
Four vaccine candidates are currently in development, three are in pre-clinical studies, and one, conducted by the United States military, is in a Phase I clinical trial.
Scoring: Based on the above analysis, the pipeline was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
Data suggest acquired protection against Campylobacter develops over time and with repeated exposure. The incidence of clinical disease falls with increasing age, particularly after five years of age 71 . Furthermore, human challenge studies demonstrate that previous infection can protect against homologous strains of bacteria. Experts confirmed that natural immunity is likely 28.
The current understanding of vaccine targets for Campylobacter is incomplete. Campylobacter pathogenesis is not well understood 71. To date, protective epitopes are not well characterised, and the degree of antigenic diversity is unclear 71. Whole cell approaches or vaccines containing Lipo-oligosaccharides (LOS) of Campylobacter raise concerns about safety, since LOS can contain N- acetyl neuraminic acid moieties that mimic human gangliosides. Antibodies induced by a potential vaccine and directed against these could cross-react with human antigens causing typical sequelae of the disease, such as reactive arthritis, Irritable Bowel Syndrome (IBS) and/or Guillain-Barre Syndrome (GBS) 71. Campylobacter expresses a polysaccharide capsule, which could provide a vaccine target based on similar conjugated polysaccharide vaccines. A capsular conjugate is the target chosen by the United States military for a vaccine currently in early clinical development.
Scoring: Based on the above analysis, pathogen biology was categorised as medium (score of 1 out of 2).
Pre-clinical and clinical R&D
Simple but predictive small animal or in vitro models are currently lacking for Campylobacter. However, experts expect that a zinc-deficient mouse model developed for ETEC is also useful for Campylobacter 72. Larger animal models that are also natural hosts of the disease such as chickens can serve as useful pre-clinical study models 71.
Correlates of protection have not yet been defined for disease caused by Campylobacter. There is a human challenge model with a C. jejuni strain that lacks ganglioside mimicry in its LOS in place 71, but it is not yet known what degree of infection or illness increases the risk of chronic sequelae or whether colonisation alone is itself a risk. Therefore it is difficult to define the relevant endpoints for a clinical programme and whether this should be prevention of disease, prevention of infection, or prevention of colonisation (or combination of thereof) 71.
Clinical infrastructure is not likely to present issues in high resource settings 71. In low resource settings, trials may be able to use the capacities built for rotavirus vaccine testing. Where this infrastructure is available, there should be sufficient field sites, experience, and regulatory pathways to take Campylobacter vaccine studies through clinical trials 71.
Scoring: Based on the analysis described above, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
Because Campylobacter is a self-limiting illness with supportive therapy in high-income settings resulting in good outcomes, the need for a vaccine is predominantly in low- and middle-income countries. A vaccination strategy in high-income countries would likely focus on a travellers’ vaccine and vaccination of military personnel deployed to low- and middle-income countries for whom sick days are problematic when planning campaigns. In low- and middle-income countries, Campylobacter would be included in the routine neonatal vaccination programme.
Support among policy makers for a prophylactic vaccine against Campylobacter for use amongst the general public is unlikely 73. As one expert explains “there may be some justification for a Campylobacter vaccine. I always thought this would be supplementary to Shigella as a combined vaccination” 28. WHO has not voiced support for a Campylobacter vaccine, and in a status document prepared for WHO PDVAC, only a combined enteric vaccine was mentioned as feasible 71.
Scoring: Based on the above analysis, expected policy stance was categorised as medium (score of 1 out of 2).
Payer, government, or Gavi support
Payers in high-income countries are unlikely to deem a Campylobacter vaccine cost-effective if given routinely. Support for a travellers’ vaccine may be feasible, but this comprises a small target population.
The incidence of Campylobacter is higher in middle-income countries than in high-income countries, but the cost-effectiveness barrier is also higher. Therefore, support from governments and payers in these countries is unlikely.
The likely route to market in low-income countries would be through Gavi support. However, given the relatively low mortality associated with Campylobacter infection, it is unlikely to be supported as a single vaccine, but could be supported as a combination vaccine with other enteric diseases 71.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as medium (score of 1 out of 2).
Barriers to uptake
A vaccine for Campylobacter would not require a new touchpoint or new clinical practices, as it could be incorporated into existing paediatric or travellers’ vaccination schedules.
Scoring: Based on the analysis described above, barriers to uptake was categorised as fairly low (score of 1.5 out of 2).
Commercial attractiveness
The largest market for a Campylobacter vaccine would be in low- and middle- income countries, but with no clear path through Gavi, it will be difficult to gain access to this market.
Scoring: Based on the analysis described above, commercial attractiveness was categorised as low (score of 0 out of 2).
Primary recommendation
The primary recommendation is to better understand disease burden, epidemiology, and transmission. More data is needed on transmission in low- and middle-income countries, particularly to clarify whether transmission occurs through environmental pathways or from animal reservoirs before a determination can be made on whether a human vaccine should be pursued or whether alternatives, such as animal vaccination, will be the preferred approach. Furthermore, there is variability in estimates of global disease burden, for example WHO groups have produced very different incidence values through different modelling techniques, showing almost a two-fold difference in values 18,74.
Given the high levels of misdiagnosis of enteric conditions, it would be useful to have targeted studies with high quality laboratory diagnostics to establish disease burden 75. Inclusion of studies such as MAL-ED and GEMS in the next iteration of the IHME Global Burden of Disease estimates may help to resolve this problem. Finally, wider data collection would reduce the need for imputation and help to establish a more accurate burden of disease.
Secondary recommendations
One secondary recommendation is to explore alternatives to a human vaccine. Developing vaccines to prevent Campylobacter infections in chickens could reduce transmission. Poultry is a major source of Campylobacter with chicken meat in retail being contaminated in up to 98% of cases in the United States and 60-80% of cases in Europe 76. Given that chicken vaccination is used by the poultry industry to protect against several viral diseases, efforts to develop effective vaccines for chickens against Campylobacter are already underway and should be further supported. For instance, Kobierecka et al. recently reported that in ovo vaccination resulted in significant levels of protection after challenge with heterologous C. jejuni strains 77.
Improved supportive therapies for Campylobacter infections should be explored. A polymer-based oral rehydration solution has been shown to be superior to the WHO standard low osmolarity oral rehydration solution in a Cochrane Review 78. In a separate Cochrane Review, children in areas of where there is a high prevalence of zinc deficiency or malnutrition were shown to benefit from treatment with zinc 79. These interventions are inexpensive, and there are low barriers to entry for production compared to vaccines.
Other secondary recommendations include exploring combination vaccines with other enteric pathogens and supporting pre-clinical research including the exploration of potential vaccine candidates using the new zinc deficient mouse model if vaccine development is later recommended 80,81.