Cross-cutting activities
Through the process of making detailed recommendations specific to each pathogen, this report also identified knowledge gaps shared across multiple pathogens. Based upon these, several cross-cutting activities have been proposed which, if implemented, would stimulate development of vaccines for all pathogens with high levels of AMR. The focus of this chapter is limited to activities specific to the pathogens in this report. It does not cover recommendations that apply more widely to the vaccine industry.
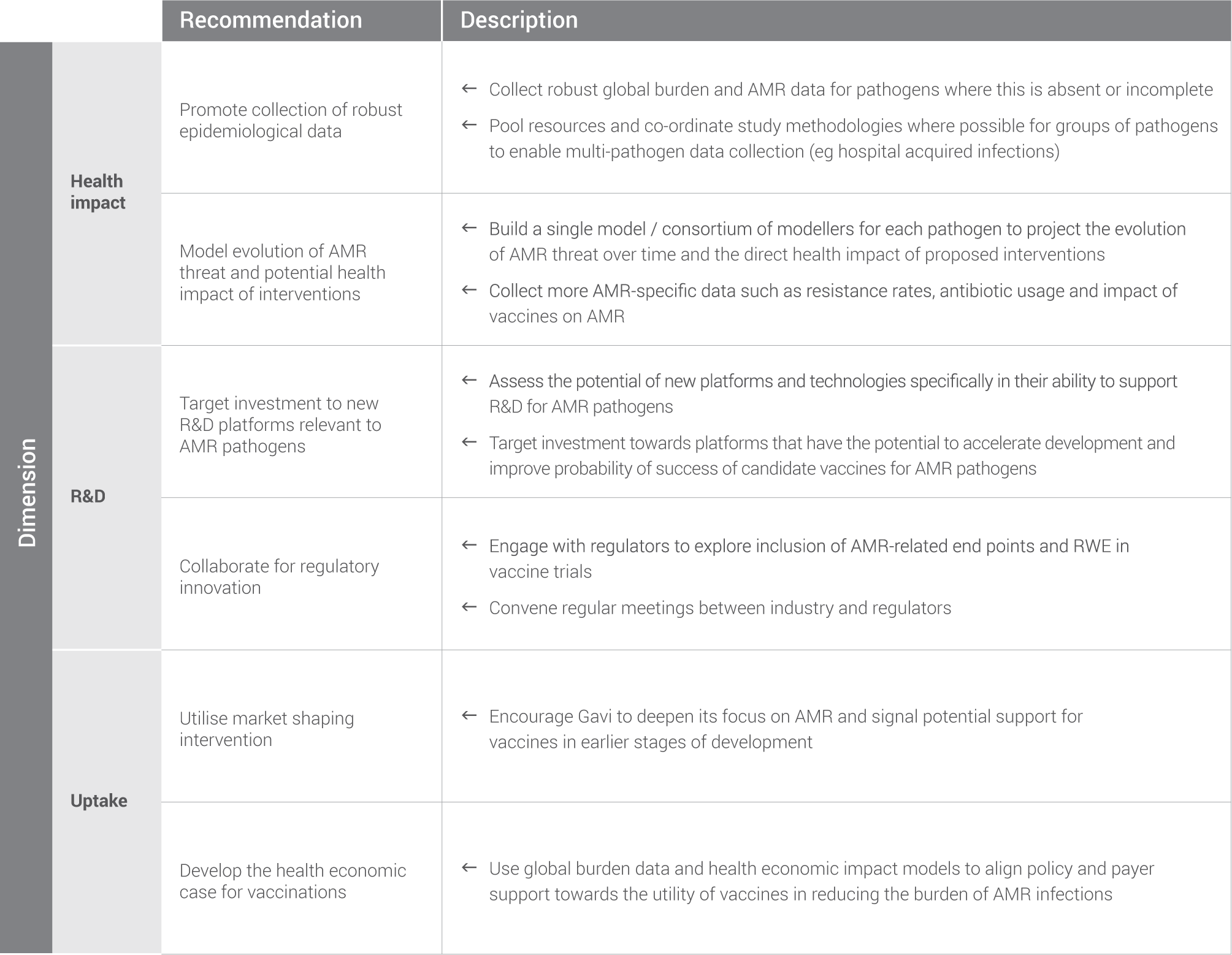
The cross-cutting activities are summarised according to the three main categories on the pathogen scorecards. They span the entire process of vaccine development – from understanding health impacts and accelerating R&D to reducing R&D costs and ensuring widespread uptake for marketed vaccines. These interventions may accelerate development across many, or all, of the AMR priority pathogens, and could represent particular areas of interest for individuals and institutions interested in impact across multiple pathogens.
CROSS-CUTTING ACTIVITIES CAN IMPROVE OUTLOOK FOR ALL AMR PRIORITY PATHOGENS IN SCOPE
Health impact
Developing a detailed understanding of the direct and indirect health impacts of a vaccine is critical to making a compelling public health investment case. In this report, the potential health impact of vaccines was assessed based upon the global burden of disease associated with the relevant pathogen. To drive positive policy changes, funding and vaccine uptake, this assessment could be enhanced by generating better data on the burden of some pathogens and employing more sophisticated dynamic modelling techniques.
Promote the collection of robust epidemiological data: In the process of compiling this report, a paucity of global burden of disease data was noted for many pathogens. Estimates were produced to fill these gaps and a detailed explanation of the methodology is included in the appendix. Going forward, the global health community would benefit from collaborative, concerted efforts to improve epidemiological knowledge. This includes knowledge for pathogens where data is already available as, at present, it represents a good estimation, but is far from exact.
The pathogens on the WHO AMR priority pathogen list are found, in differing extent, in low- middle-, and high-income countries. Collecting data on the burden of disease is especially difficult in low- and middle-income countries with poor access to healthcare and weak health surveillance infrastructure. Even in high-income countries, data quantifying the burden of disease is often limited and varies in both quality and scope. Of the pathogens included on this list, high quality data is particularly scarce for those that cause hospital-acquired infections in low and middle-income countries.
The WHO and IHME both publish regular assessments of the global burden of disease. However, this data is often provided at the level of disease (e.g., “skin infection”) and does not provide granularity at a pathogen level (e.g., “S. aureus skin infection”). Global burden data exists for S. pneumoniae, M. tuberculosis, N. gonorrhoeae, Shigella, Campylobacter, Salmonella, E. coli (enteric) and H. influenzae. Further, data on the global burden of enteric disease has been gathered through a number of well-funded, multi-country, multi-pathogen studies. These include both the Global Enteric Multicenter Study (GEMS) 16, the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health study (MAL-ED) 17, and the World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010 18. The challenging nature of gathering data of this kind has led experts to express concerns about the quality of current enteric disease burden estimates. This highlights that even where data exists, it is often imperfect and efforts to improve its quality should continue. Several outstanding questions have been highlighted including the relative importance of different transmission routes for Campylobacter and the regional burden of enteric E. coli and non-typhoidal Salmonella.
However, global burden data does not exist for infections caused by S. aureus, H. pylori, A. baumannii, P. aeruginosa, Enterobacteriaceae, K. pneumoniae or E. faecium (see methodology appendix for more information on how estimations were made).
Many epidemiological studies focus on either a single pathogen or a single geography. Whilst acknowledging that expanding studies to cover more than one country can be challenging, the existing multi-country, multi-pathogen studies on enteric disease prove that this is possible. Additional multicentre studies would provide scale to research efforts and may make data more robust.
Model the evolution of AMR and potential health impact of interventions: The assessment in this report is based upon the most up-to-date available data for each pathogen and provides a good starting point for the analysis of health impact. However, AMR is a complex and constantly evolving threat. In addition, vaccine efficacy, pathogen transmission dynamics, and vaccine uptake have not been factored in to the assessment.
Whilst these simplifications still allow for a useful comparison of the pathogens within this report, a more comprehensive model, perhaps derived through a consortium effort, could allow for the evaluation of the contribution of different interventions (e.g. vaccines, sanitation, and therapeutics) to reducing the prevalence of drug resistance in individual pathogens. A first step may be to harmonise modelling strategies, methodologies and assumptions so that more useful comparisons can be drawn amongst disparate models.
More definitive pathogen models, or consortia of models, would serve as a common resource for the global health community. These models would also be useful to support policy making and funding decisions once vaccines are licensed.
In addition to the need for robust data on the global burden of disease (discussed above), there are three inputs which would be critical for the modelling process:
- Data on the antibiotic usage associated with a pathogen. This would require a significant international effort to collect suitable input data. Whilst some data exists on global antibiotic usage, going forward this data should be linked to specific pathogens, and efforts should be made to encourage others to follow the process and record data in a standardised manner.
- Data on the prevalence of resistant strains for each pathogen. The recent announcement of an “AMR project” to integrate such data as part of IHME’s Global Burden of Disease dataset is a very positive step forward in these efforts 19. The Wellcome Trust is working closely with the IHME and co-funding this work.
- Data on the impact of vaccines in reducing antibiotic usage and the prevalence of resistant strains. Limited data is available for the impact of S. pneumoniae and H. influenzae vaccines on these outcomes, but should be collected for other marketed vaccines.
Research and development
The challenges of bringing a vaccine to market are well documented and this report does not seek to directly tackle this wide-ranging topic. Rather, it aims to highlight activities that could address several of the pathogens included in this report, with a specific focus on reducing the AMR threat.
Target investment to new R&D platforms relevant to AMR pathogens: There are a range of promising new technologies, platforms and pre-clinical approaches that could aid vaccine development for the pathogens listed in this report. Examples include DNA and RNA vaccines, viral vectors, nanoparticles, novel delivery/administration technologies, and modular manufacturing platforms. These platforms have the potential to both significantly lower vaccine manufacturing costs and to facilitate development of polyvalent vaccines – two key issues in R&D for many AMR priority pathogens.
Not all new platforms will be equally useful at addressing the pathogens in this report – some may have wide ranging benefits with regards to vaccine development as a whole, but have little specific utility in addressing the AMR threat. In order to maximise impact, new platforms should be assessed against the unique requirements of pathogens with high levels of AMR. For example, many of these pathogens exhibit high antigenic variation. This issue complicates vaccine development and would be well addressed by the use of new platforms that allow for multiple antigens to be targeted simultaneously at a low cost. Experts expressed optimism that vector-based platforms, such as DNA vaccines, viral vector vaccines, and novel conjugation techniques could all provide a step-change in the ability to target multiple antigens in a single vaccine.
Collaborate for regulatory innovation: In North America and Europe, the impact of vaccines on AMR and antibiotic prescribing is not currently considered as a reportable outcome by any major regulatory body. Inclusion of AMR and antibiotic prescribing as a reportable outcome would increase the evidence-base supporting the use of vaccines to tackle AMR 20.
There has been a growing interest in the use of real-world evidence (RWE) to support pharmaceutical development 21. Efforts in vaccine development are currently limited to proof-of-concept studies22 but may provide a more affordable method of collecting data in the future.
Experts also expressed the opinion that additional opportunities for industry and regulators to convene would help foster closer ties. This could take the form of individual companies meeting with their individual regulator or meetings convened between industry and multiple regulatory agencies. These convenings should be international, where possible, to share expertise and harmonise processes where this is appropriate.
Uptake
Vaccines against AMR priority pathogens face particular barriers to uptake, owing either to the concentration of prevalence in low- and middle-income countries or the challenging economics of vaccinating small target populations. This is discussed in detail in the chapter on pathogen comparisons. However, across groups of pathogens there are common recommendations:
Continue to utilise and improve market shaping interventions where needed: For pathogens where there is a clear commercial vaccine market in high-income countries – for example, S. aureus – early discussion with payers and policy makers would likely improve vaccine uptake. Improving uptake is unlikely to require market shaping interventions. However, more robust burden of disease data would be beneficial, as discussed earlier in this chapter. Vaccines targeting pathogens where there is a clear Gavi market and significant public / philanthropic funding of R&D, such as for M. tuberculosis, are also unlikely to require any further market shaping interventions.
Many of the pathogens in this report predominantly impact low- and middle-income countries where Gavi support is essential for vaccination uptake. However, some potential vaccines are borderline or unlikely candidates for Gavi support. Gavi has recently incorporated impact on reducing AMR as a criteria in its Vaccine Investment Strategy (VIS) which determines the vaccines that are supported in its portfolio. Gavi’s continuing shift to place more emphasis on reducing AMR will favour vaccine development for pathogens with high levels of AMR. In order to support pathogens where the pipeline is at a much earlier stage, there may be some benefit from Gavi indicating its interest in pathogens beyond the current five-year window of their VIS.
Develop the health economic case for vaccination: Gathering robust data on burden of disease is a key recommendation earlier in this chapter and is absolutely essential to making the health economic case for vaccination. This data can then be translated into both direct costs to the patient and health care system, as well as indirect costs related to the economic impact of a pathogen. These indirect costs include loss of GDP driven by lower productivity, premature deaths, and the costs of informal care (e.g. individuals caring for ill relatives). Modelling these costs on a national or regional level is a significant undertaking, but is crucial to drive positive policy change.
In addition to the metrics commonly included in health economic evaluations, the assessment of vaccines for pathogens with high levels of AMR could also include consideration of the potential cost savings associated with reducing AMR in the wider population. Frameworks for incorporating this value would need to be established.