Escherichia coli (urinary)
Escherichia coli (E. coli) is a gut commensal that is part of the Enterobacteriaceae family, but is considered separately in this report because the higher burden of disease merits individual assessment. E. coli can be divided into multiple different subtypes. To facilitate a useful comparison two E. coli groupings were created – E. coli (enteric) representing those subtypes causing enteric infections (ETEC, EPEC, EIEC, EHEC and EAEC) and E. coli (urinary) representing UPEC infections.
E. coli can cause urinary tract infections (UTIs). UTIs caused by E. coli are referred to as urinary E. coli in this report. Urinary E. coli has a high incidence and may be attractive for targeted vaccination in high-income countries, despite relatively low mortality and morbidity. There are candidates in clinical development but antigen selection remains an ongoing challenge. Payers in high-income countries are likely to support vaccination for specific sub-populations at high risk. This approach is likely to be cost-effective due to the high cost of UTIs.
Urinary E. coli falls into a cluster of pathogens for which advancing early R&D is the priority. The primary recommendation is to advance pre-clinical research. The secondary recommendations are to explore alternative treatment and prevention strategies and to better understand the disease burden, epidemiology and transmission.
E. coli is a Gram-negative commensal bacterium that predominantly causes community-acquired infections, but also can cause hospital-acquired infections. E. coli is part of the Enterobacteriaceae family. In this assessment, E. coli is considered separately because of its high incidence relative to other Enterobacteriaceae family members.
E. coli is part of the normal gut flora, but several pathotypes cause pathogenesis 131. Urinary symptoms are caused by uropathogenic E. coli (UPEC) 153, which belongs to a family of E. coli pathotypes that cause infection outside of the gut, known as extraintestinal pathogenic E. coli (ExPEC) 154. A large variety of UPEC virulence genes exist andit has been suggested that there are multiple UPEC pathotypes 155. Transmission of UPEC occurs through bowel contamination or sexual activity 153,156.
Clinical features of urinary E. coli infection include urinary frequency, dysuria, urgency, loin pain, and fever 118. In the community setting, women are more likely to contract UTIs than men 157. In the hospital setting, catheterised patients and patients undergoing urological procedures, including transurethral procedures and transrectal prostate biopsies, are at greatest risk of urinary E. coli infections 158. The geographic distribution of urinary E. coli infection is poorly characterised. In particular, insufficient data from low-income countries exists to determine differences in regional burdens 159.
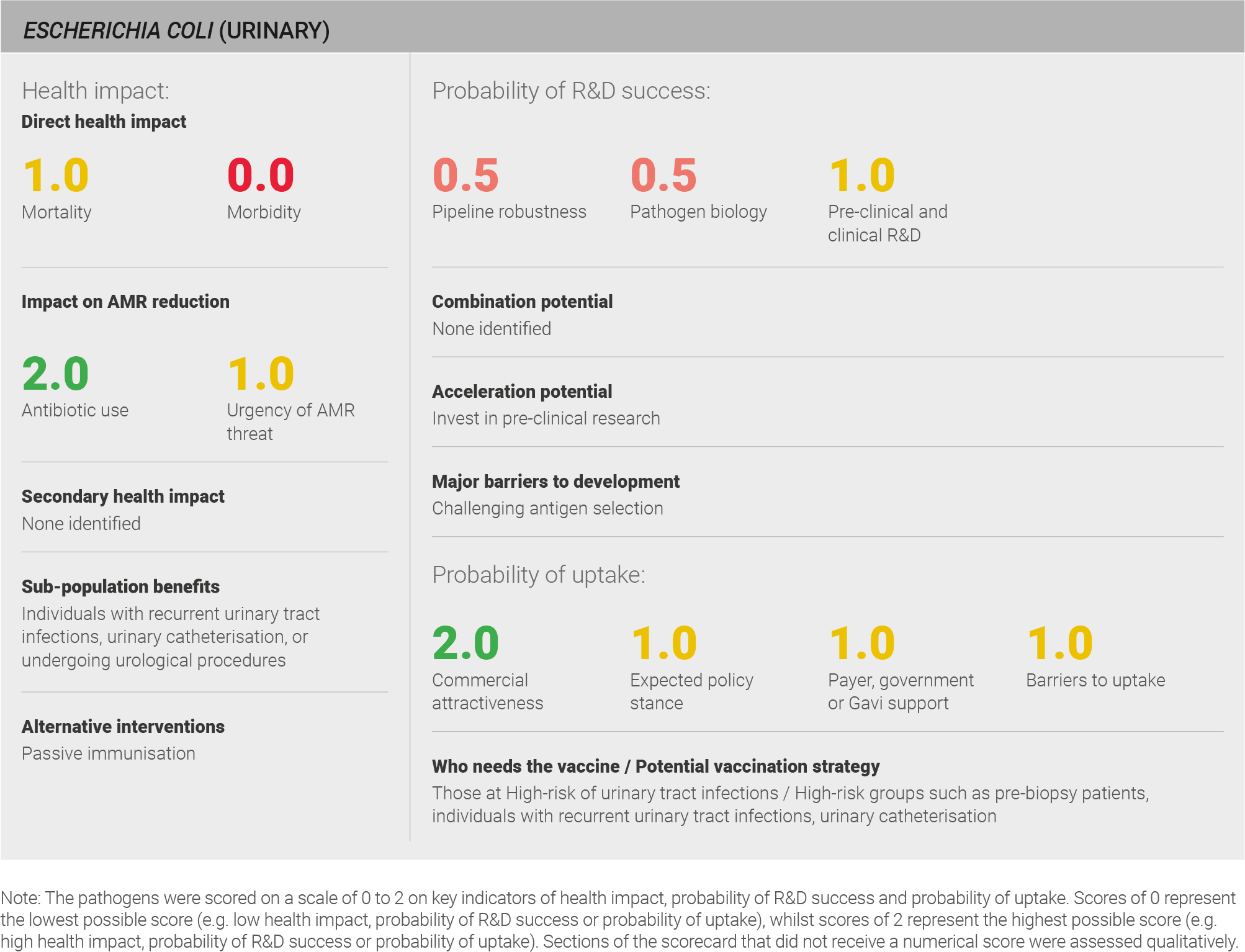
Direct health impact
Robust global data on disease burden is not available. Urinary E. coli infections are not reported by the WHO or IHME and no publications were found in the literature that report the global burden of these infections. Some data on burden of disease exists in high-income countries, such as the United States, but data is scarce for low- and middle-income countries 159. Experts are also uncertain as to the burden of disease 28.
A review of the literature suggests that urinary E. coli causes significant disease burden and is responsible for ~70% of UTIs globally 82. Given limited data at a global level and uncertainty among experts regarding disease burden, confidence in this estimate is relatively low. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was scored as medium (score of 1 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
UTIs disproportionately affect women, and women who experience recurrent UTIs would benefit from a vaccine. Subpopulations likely to benefit from a vaccine include patients undergoing urological procedures and patients with long term indwelling catheters 157,158.
Antibiotic use
Recommended antibiotic treatment regimens differ within and between countries, in part reflecting local resistance profiles. Regimens typically involve a seven-day oral course of an antibiotic such as nitrofurantoin or trimethoprim, or other first line agent 26,118.
Scoring: Based on the above analysis, antibiotic use was categorised as high (score of 2 out of 2). This estimate is based on an annual incidence of ~250 million urinary E. coli cases treated with a seven day course of antibiotics
Urgency of AMR threat
Both the WHO and CDC have expressed concern about antibiotic treatments for Enterobacteriaceae, and E. coli falls within this family. The WHO has listed the Enterobacteriaceae group as a ‘critical’ priority for R&D regarding new antibiotics 32. The CDC has listed carbapenem-resistant Enterobacteriaceae as an ‘urgent’ threat in its list of biggest threats from AMR and extended spectrum beta-lactamase-producing Enterobacteriaceae s as a ‘serious’ threat 7. However, fluoroquinolone resistance rates for E. coli are less than 10% in much of North America and Europe, albeit with a trend of increasing resistance, notably from sequence type 131 118,125,126,160. Compared to other bacteria in the Enterobacteriaceae family, there is a lower frequency of AMR, and resistance is typically limited to fewer antibiotics.
Scoring: Based on the above analysis, the urgency of AMR threat was categorised as medium (score of 1 out of 2).
Pipeline robustness
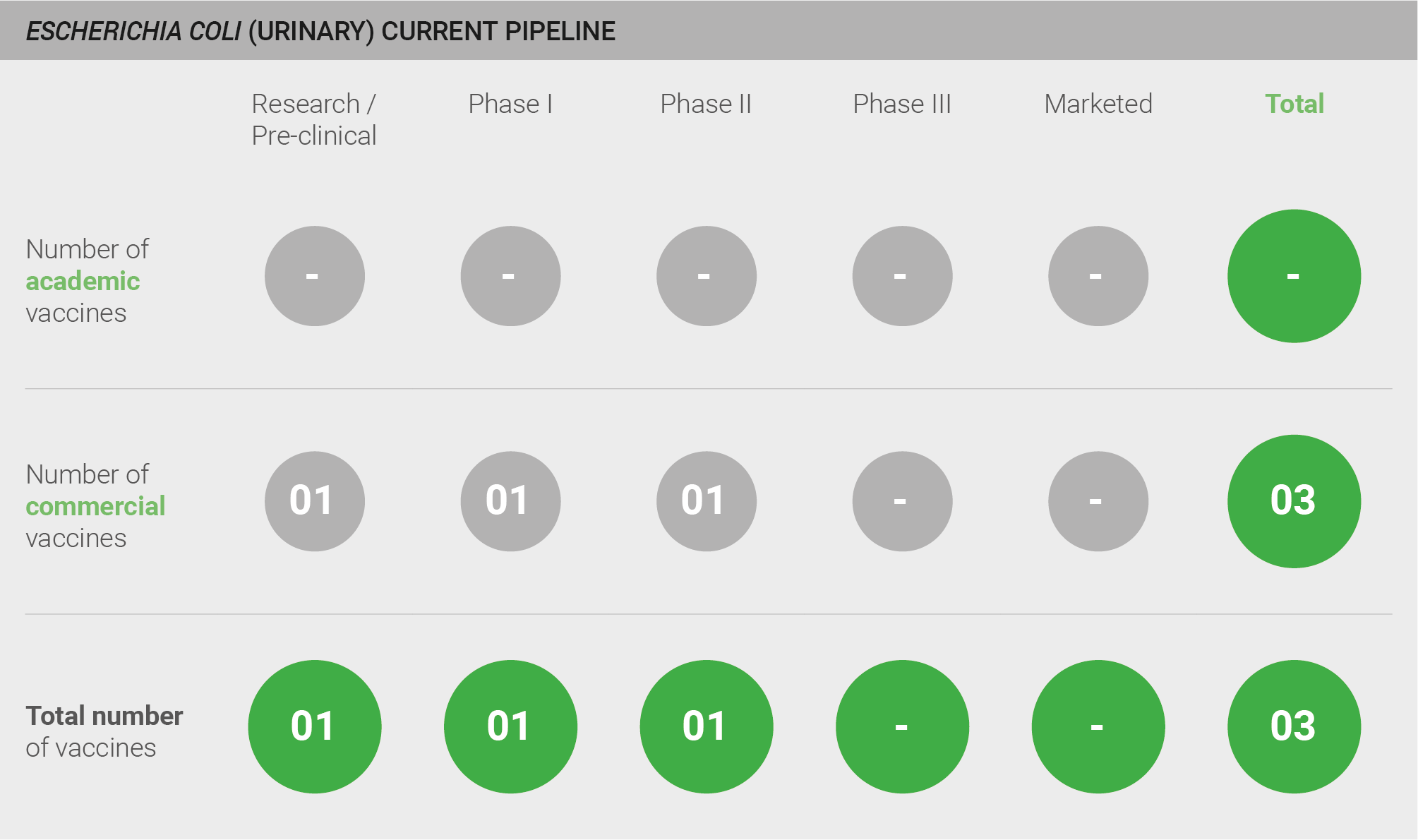
There is very little ongoing vaccine development for urinary E. coli. One candidate is in pre-clinical development, and two vaccines are reported to be in clinical development.
The Phase II vaccine targets ExPEC and is known as ExPEC4V or JNJ-63871860. ExPEC4V was originally produced by GlycoVaxyn (GlaxoSmithKline) and is now being co-developed by GlycoVaxyn and Janssen. It is a bioconjugate vaccine that uses GlycoVaxyn’s proprietary glycosylation platform against E. coli infection 161. ExPEC4V targets the O-antigen of four ExPEC serotypes: O1A, O2, O6A, and O25B 162.
Data from two Phase I trials are available in the public domain. ExPEC4V was well-tolerated and elicited an immunogenic response in a Phase I trial in healthy Japanese participants 163. Another Phase I trial was conducted in Switzerland in healthy women with a history of recurrent UTIs 161. In this trial, ExPEC4V was safe and well-tolerated, and elicited strong, durable, and functional immune responses. Phase II trials are ongoing in adults in the United States.
Scoring: Based on the above analysis, the pipeline was categorised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
The high incidence of recurrent and chronic UTIs suggests a lack of natural immunity 157, especially as recurrent UTIs are often caused by the same pathogen as the original UTI 164.
Experimental work suggests that the inflammatory response mounted to urinary E. coli may itself adversely affect the adaptive immune response 165,166.
Although the genomes of UPEC frequently encode many more virulence factors than commensal E. coli, there is no defined core set of virulence factors that clearly differentiates UPEC from commensal E. coli 167. Some initial efforts to develop a vaccine focused on surface polysaccharides; however, these antigens are highly diverse, making them a challenging target for a vaccine designed to achieve broad coverage. Overall, 167 O serogroup antigens have been identified, and the K serogroup is comprised of more than 80 members. However,it is likely that 10-12 O serotypes account for at least 90% of meningitis isolates and >60% of bacteraemia isolates 168. Even with a more limited number of serotypes, designing a broadly protective UPEC vaccine against these serotypes is challenging 98. Furthermore, these antigens are poorly immunogenic, as some are camouflaged from the adaptive immune system due to structural similarities with host antigens 98.
Other potential targets include fimbrial adhesins, toxins, and iron acquisition system-based antigens. Thus far, only a small subset of fimbrial adhesins has been evaluated for use in a UTI vaccine (P, Dr and type 1 fimbriae) 98. Many toxins have been associated with UTI symptom severity but none seem to be required for infection 98. Iron acquisition system-based antigens include outer membrane iron compound receptors 98 and iron-binding siderophores 98. These molecules are required for bacterial growth in the host and could represent an appealing target for vaccine development.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Expected policy stance
A vaccination strategy for urinary E. coli would target high-risk populations. These populations would likely include patients undergoing pre-prostate biopsy, as well as patients with recurrent UTIs or long-term catheters.
The high incidence and significant morbidity and mortality of urinary E. coli infection in some select sub-populations suggest that a vaccine is likely to have policy support. The frequent use of antibiotics to control urinary E. coli infection also prompts interest in preventing infections, as one expert notes, “vaccination is attractive as a non-antibiotic means of controlling E. coli urinary tract infections” 28. The existence of potentially large, defined target populations also suggests likely support, as explained by a policy expert “at least 50% of men on earth will undergo prostate biopsy at some stage so this could be a target population” 28.
However, significant challenges exist in developing vaccination strategies for key target populations (discussed in more detail section “Barriers to uptake”) that could prompt some caution on the part of policy makers.
Scoring: Based on the analysis described above, expected policy stance is categorised as medium (score of 1 out of 2)
Payer, government or Gavi support
Payers in high-income countries are likely to support vaccination for specific sub-populations at high risk, given the probable cost-effectiveness of a targeted vaccination strategy. The annual cost of UTIs, including healthcare and time off work, is approximately US$3.5 billion per year in the United States 82. One potential target population is patients prior to prostate cancer biopsy, as an alternative to antibiotic prophylaxis 171. An estimated 1.2 million prostate biopsies are conducted per year in the United States alone 1, and rates of the procedure are similar in other high-income countries. Prophylactic antibiotic use is frequently standard practice for biopsy patients 172. The infection rate after biopsy is ~2% and carries the risk of sepsis and mortality from infection 2. There may also be support for vaccination amongst patients with recurrent UTIs. UTIs are common and ~25% of women experience recurrent UTI, with many experiencing recurrence despite prophylactic antibiotics 173.
In middle-income countries, the cost-effectiveness threshold for a urinary E. coli vaccine will likely be higher because of lower healthcare spending per person. Therefore, urinary E. coli vaccines may not be a priority within these health systems. In low-income countries, Gavi is unlikely to support a vaccine for urinary E. coli due to low associated mortality.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as medium (score of 1 out of 2).
Barriers to uptake
For pre-prostate biopsy, vaccination could likely be incorporated into the pre-procedure pathway. The logistics of administration would generally allow sufficient time for maximal immune response.
Targeting patients with recurrent UTIs would require greater planning and investment. Key challenges would include the need to engage strongly with guideline setting bodies and key opinion leaders to establish thresholds for when the vaccine would be recommended.
In patients with long-term catheters, catheter associated UTIs account for ~20% of all hospital-acquired infections and ~50% of all infections in long term care facilities 174. There are likely to be few barriers to uptake in this population as administration of a vaccine could be easily scheduled as part of the catheterisation pathway. Patients and clinicians and likely to be keen to avoid infections, which are a common complication with indwelling catheters.
Scoring: Based on the above analysis, barriers to uptake was categorised as medium (score of 1 out of 2).
Commercial attractiveness
A urinary E. coli vaccine may be commercially attractive given the potential utility in several sub-populations in high-income countries and medium likelihood of payer, government, or Gavi support. Each affected sub-population currently has a high cost of treatment from UTIs.
Scoring: Based on the above analysis, commercial attractiveness was categorised as medium (score of 1 out of 2).
Primary recommendation
The primary recommendation is to invest in pre-clinical research. Further research into vaccine targets, especially identification of factors that differentiate urinary E. coli from E. coli found as a gut commensal, would facilitate development of a vaccine specifically targeting urinary E. coli.
Substantial diversity exists within classes of UPEC virulence factors, which likely contributes to the difficulty in finding antigens that provide broad coverage against UPEC. In order to discover conserved antigens, further understanding of UPEC pathogenesis and the host mucosal immune response to infection will be necessary 98. Identifying antigens that can be included in vaccines that target virulence factors beyond lipopolysaccharides is also likely to be useful 98.
Pre-clinical research should also seek to better understand the potential effect of vaccines on gastrointestinal flora. The gut microbiome is recognised as an important actor in a range of health outcomes, from mood to body weight 175,176 As E. coli is a key gut commensal, it is important to establish the presence of any disruption to the microbiome from a vaccine. Initial animal studies regarding the microbiome have been undertaken for Enterotoxogenic E. coli (ETEC); however, such work has yet to be pursued for UPEC 177.
Secondary recommendations
A better understanding of disease burden should be pursued through pathogen level epidemiological studies. The burden of disease and regional breakdowns provide important information for determining vaccination strategy and assessing cost-effectiveness. These decisions impact subsequent commercial decision making regarding whether to invest in vaccine development.
There is no single source of information that presents a global view of the incidence, morbidity and mortality caused by urinary E. coli infection. For urinary E. coli, understanding the proportion of hospital-acquired UTIs arising post-surgery would also aid in determining the feasibility of an elective surgery vaccination strategy. There are no regional breakdowns of the urinary E. coli disease burden, and there is a particular paucity of information from low- and middle-income countries.
Alternative treatments should also be explored. An alternative prevention strategy in pre-surgical groups would be the use of monoclonal antibodies. The advantage is that if a procedure needed to be carried out urgently, monoclonal antibodies would provide rapid protection. However, monoclonal antibodies would not provide sustained protection in recurrent UTI and or long-term catheterised patients. Further, monoclonal antibody approaches face many of the same development challenges as vaccines.