Mycobacterium tuberculosis
Tuberculosis (TB) is the world’s deadliest infectious disease and is responsible for ~1.3 million deaths annually. The current BCG vaccine is widely used but has variable efficacy. There is a strong case for developing an effective vaccine against Mycobacterium tuberculosis (M. tuberculosis) given its health impact and AMR threat. However, pathogen biology and host immune response to M. tuberculosis are not well understood and clinical trials are challenging to design and conduct due to the lack of reliable correlates of immune protection, the difficulty of controlled human infection studies and lack of trial infrastructure in rural areas. Given the high global disease burden, vaccine uptake is likely to be high if a highly effective vaccine is developed.
M. tuberculosis falls into a cluster of pathogens for which advancing early R&D is the priority. The primary recommendation is to improve the translatability of pre-clinical research. The secondary recommendation is to support pre-clinical research.
M. tuberculosis is an aerobic, non-motile bacillus that can be acquired in community or hospital settings. Due to the high lipid content in its cell wall, M. tuberculosis does not retain any common bacteriological stain and is therefore not considered to belong to either Gram-positive or Gram-negative categories 260.
M. tuberculosis predominantly causes pulmonary disease. In immunocompromised patients, M. tuberculosis can affect multiple other systems including gastrointestinal, central nervous (CNS), and genitourinary systems, and can also affect bones 261.
The primary mode of transmission is by airborne droplet. Symptoms of TB vary by lesion location, but often include fever, night sweats and weight loss, and pulmonary infection is associated with dyspnoea and chest pain. Gastrointestinal infections cause abdominal pain, nausea, vomiting, and diarrhoea. CNS infection can cause headache, fever, neck stiffness, and neurological deficits.
Incidence of TB is estimated at nearly 10 million cases per year 31 and is particularly common in South East Asia and Africa. High-risk groups include persons who have been recently infected with M. tuberculosis and immunocompromised persons 262. Globally, TB is also responsible for between 6% and 15% of maternal mortality 263.
The BCG vaccine is the only marketed vaccine for M. tuberculosis; the vaccine is produced by at least nine pharmaceutical companies 40 and global vaccine coverage is estimated at ~90% 264. The vaccine is approximately 20% effective in preventing primary infection but can reach up to 80% effectiveness depending on location. It is also approximately 60% effective in preventing progression to active disease in those infected. Furthermore, BCG efficacy appears to be variable, ranging from substantial protection shown in the UK MRC trial to the absence of clinically important benefit in a trial conducted in South India 259.
Direct health impact
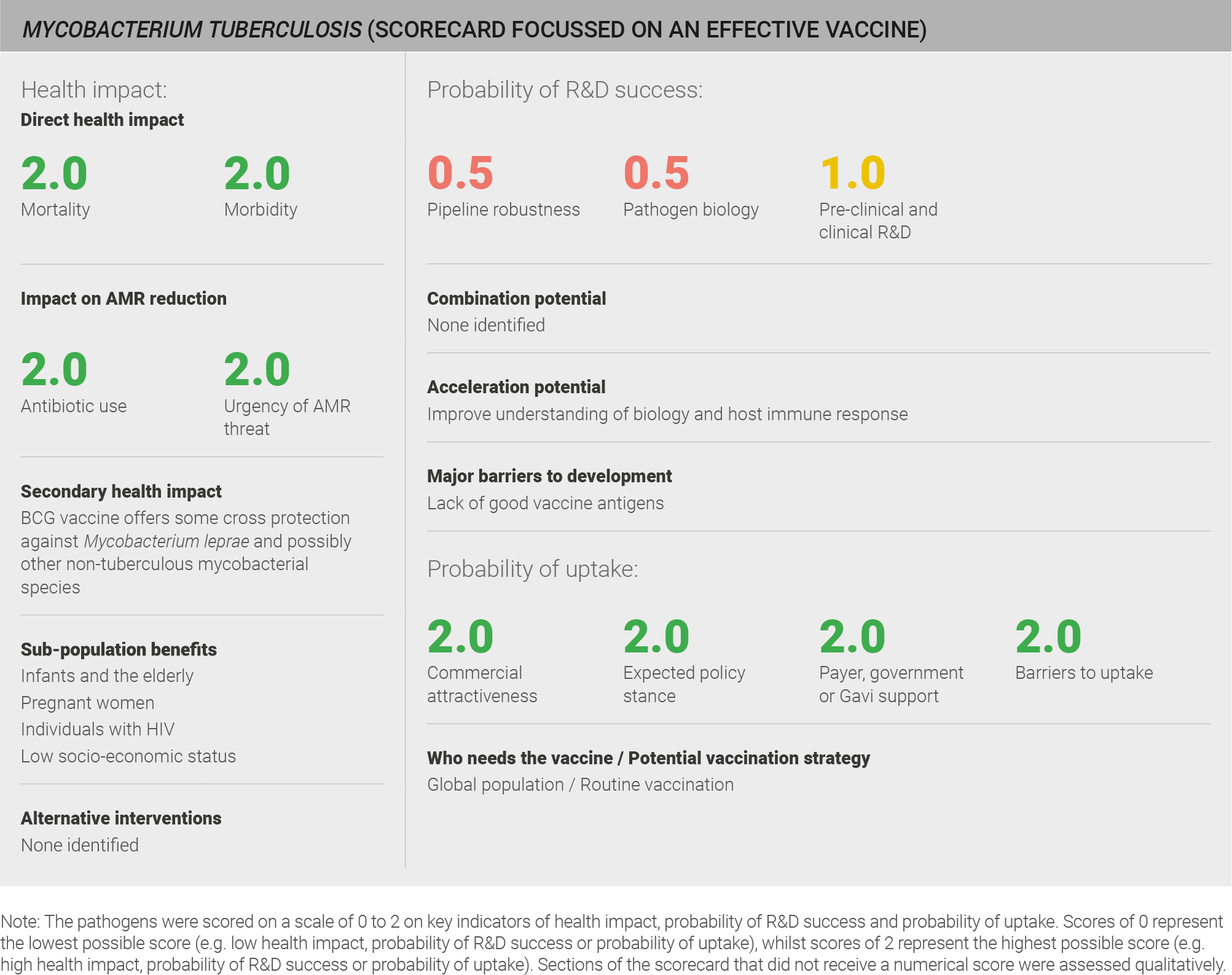
Data on morbidity was available from the IHME 2016 estimates, and data on mortality was available from the WHO estimates for 2016. Both of these data sources have a defined methodology and are used in the global health community. TB has a relatively low incidence compared to other pathogens in the evaluation set, but causes high morbidity (~3.3 million years lived with disability per year) 31 and mortality (~1.7 million deaths per year) 265 globally. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was categorised as high (score of 2 out of 2) and morbidity was categorised as high (score of 2 out of 2).
Secondary health impact
A vaccine against M. tuberculosis may provide cross-protection against Mycobacterium leprae and possibly other mycobacterial species 258.
Sub-population benefits
Infants and young children are at specific risk of developing severe, disseminated disease, often presenting as TB meningitis or miliary TB; these infections often lead to death. Extra-pulmonary TB occurs in approximately 20-30% of all cases in children 266. An efficacious vaccine would also be of particular benefit for people with low socioeconomic status, pregnant women, and HIV-infected individuals, all of whom are at elevated risk of contracting TB.
Antibiotic use
Treatment of TB requires several antibiotic drugs given simultaneously over at least a six month course of treatment, depending on age, overall health, possible drug resistance, the form of TB, and the infection’s location in the body 267. Typical antibiotics used to treat TB include isoniazid, rifampicin, ethambutol, and pyrazinamide. In patients known or suspected to have drug-resistant M. tuberculosis infections, a combination of specific antibiotics (e.g., fluoroquinolones, amikacin, kanamycin, capreomycin) is generally used for 20-30 months.
Scoring: Based on the above analysis, antibiotic use was categorised as high (score of 2 out of 2). Estimate based on an annual incidence of ~ nine million TB cases treated with an eight month course of antibiotics (Isoniazid and Raifampicin for six months; Pyrazinamide and Ethambutol for two months).
Urgency of AMR threat
Both the WHO and CDC have expressed concern about antibiotic treatments for M. tuberculosis. M. tuberculosis is listed as ‘critical’ in the WHO priority list of research and development for new antibiotics 31 and as a ‘serious’ threat in the CDC list of biggest threats from AMR 7. M. tuberculosis resistant to all antibiotics was first reported in 2009 1 and extensively drug-resistant M. tuberculosis (XDR-TB) was reported by 123 WHO member states by the end of 2016 2. Approximately 0.5 million new cases of multi-drug resistant M. tuberculosis were reported worldwide in 2016 2.
Scoring: Based on the above analysis, AMR threat was categorised as high (score of 2 out of 2).
In this assessment “Pathogen biology” and “Pre-clinical and clinical R&D” are scored assuming a highly efficacious vaccine for infants as well as adults that prevents sustained de novo infection. For “Pipeline robustness” all TB vaccines in development are counted, but the score is adjusted via qualitative pipeline assessment.
Pipeline robustness
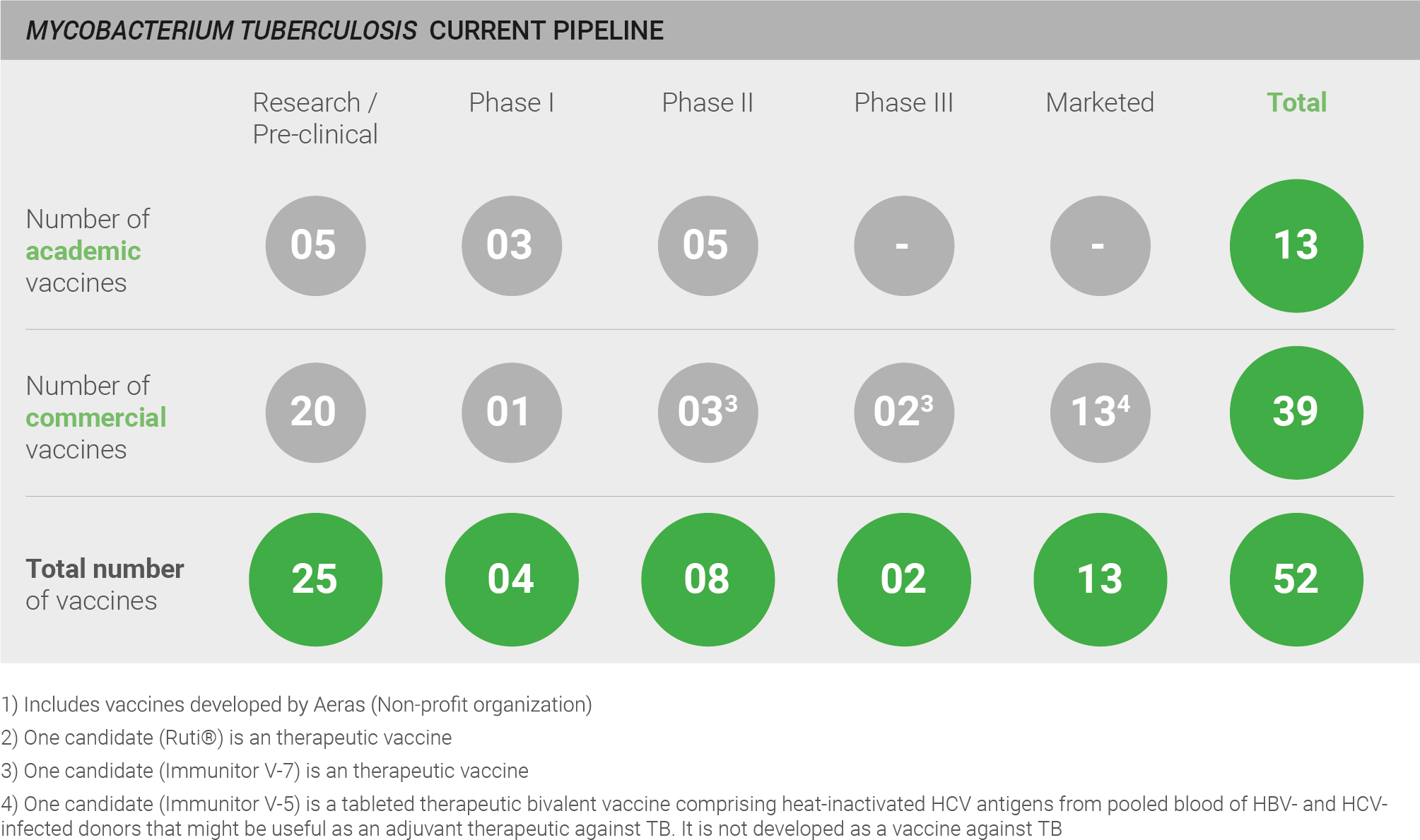
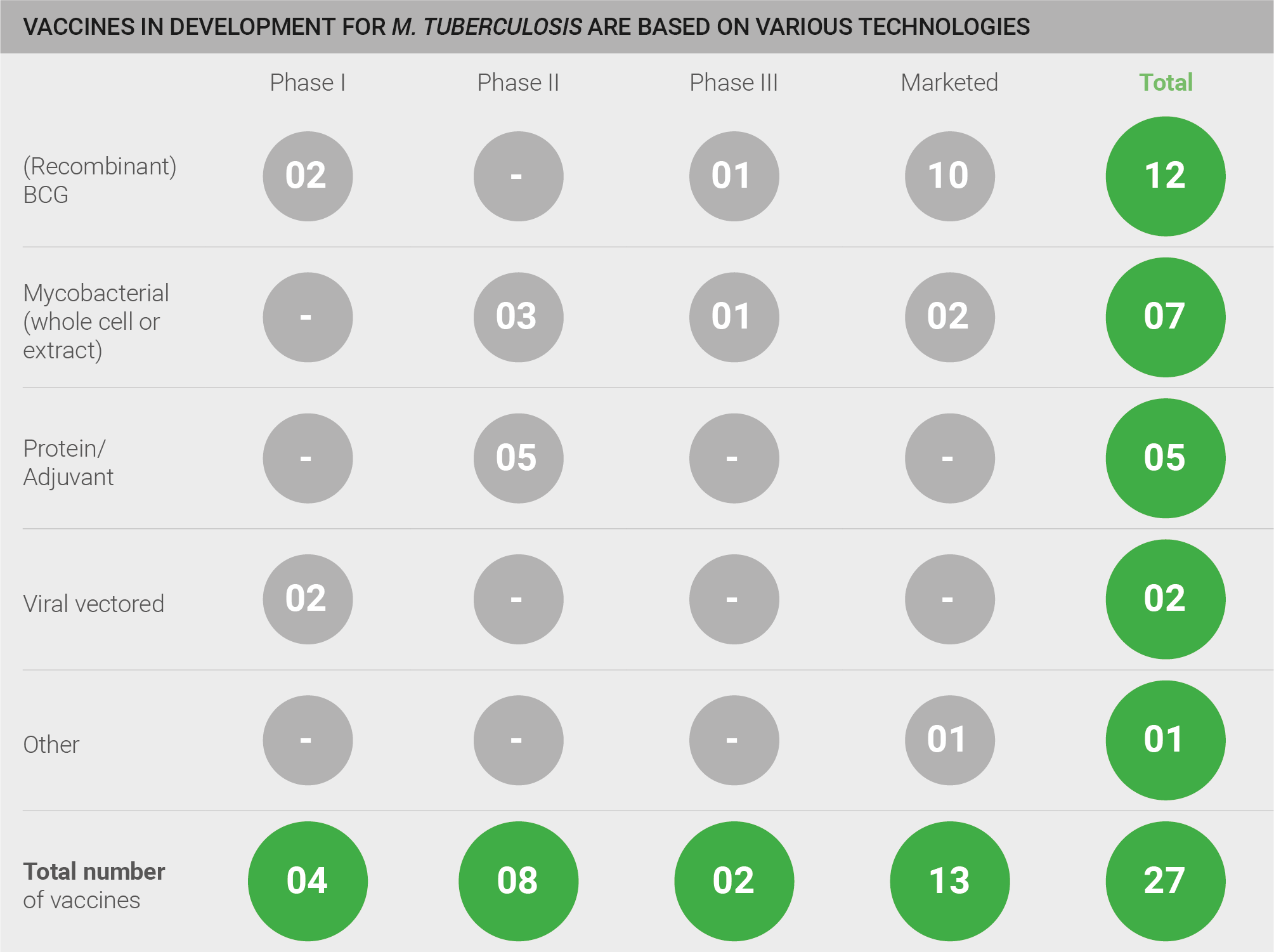
The pipeline for the development of vaccines against M. tuberculosis is highly active, with a total of 52 vaccines: 25 of these are in pre-clinical development; four are in Phase I, eight in Phase II, two in Phase III, and 13 vaccines are marketed. However, a qualitative assessment of the pipeline is also necessary for a full understanding of pipeline robustness. Whilst BCG and mycobacterial vaccines are marketed and in clinical development, only a limited number of truly novel protein/adjuvant and viral vector vaccines are in development, and those are currently in pre-clinical and earlier stage trials. Thus, a truly novel efficacious vaccine is not yet close to fruition. One exception is a clinical candidate developed by GSK (M72) which has recently completed an efficacy trial and will read out shortly 28.
Exhibit: Vaccines in development for TB are based on various technologies
Furthermore, not all vaccines against M. tuberculosis are developed with the goal of achieving high efficacy in the prevention of sustained de novo infections; candidates may be developed with narrower goals in mind, including the prevention of TB in adolescents and adults; as a replacement for BCG for early life immunisation; as BCG boosters; for vaccination of TB patients after treatment to prevent disease recurrence; or as immunotherapeutic adjuncts to drug therapy intended to reduce treatment duration 268. One expert noted that therapeutic adjuncts are a particularly valuable approach as “therapeutic vaccines [given together with antibiotics] make a lot of sense, as they reduce treatment time and by doing so compliance [and thereby reduce development of AMR]” 28.
Experts also consistently acknowledge the need for a vaccine that effectively prevents de novo TB infection in a broad population but are divided over the feasibility of achieving this ambitious goal 28. One expert offered the following assessment of feasibility, explaining “In tuberculosis the potential for a vaccine is very high but we are at least a decade away from creating a vaccine other than BCG in children,” 28 and another stated, “I think we are not going to see anything novel in the next 10 years other than the new BCG from Serum Institute of India. At best this will replace BCG as an early life vaccine. [And it will take] 15-20 years to know if it has [an] effect” 28. Many experts doubt the potential for success of current vaccine candidates, explaining “BCG variants are not impressive, some subunit vaccines will report shortly but I am sceptical [about the results]” 28. However, others remain interested in novel approaches, noting “there could be novel concepts, for example whole inactivated M. tuberculosis showed proof of concept in HIV infected people in Tanzania” 28.
Scoring: Based on the above analysis, pipeline robustness was characterised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
Some level of natural immunity against M. tuberculosis does exist 269,270; however, protective immunity is a subject of debate in the TB research community. Most individuals develop partial immunity post-infection and are able to control but not eliminate the pathogen. M. tuberculosis is highly prevalent, with latent M. tuberculosis infection affecting approximately 25% of the worldwide population 271. However, only a small percentage of infected and immune-competent individuals – approximately 5-10% – develop active disease.
Despite extensive research on M. tuberculosis biology, no target antigen has been identified and proven to be protective to date. It is possible that protection may not be easily measured by a specific antibody titre or an absolute number of protective immune cells against specific antigens, but rather by the relative ratio of protective and suppressive immune cells that recognize M. tuberculosis antigens. Hence, the goal of immunisation may not only include identifying antigens that will promote an immune response, but also directing the ratio of immune cells to the most effective balance 270.
Some experts point out that M. tuberculosis might have evolved to ensure T-cell recognition 28. Known T-cell epitopes are hyper-conserved and represent some of the most conserved regions of the M. tuberculosis genome. This may indicate that T-cell epitope conservation is critical to survival and spread of M. tuberculosis. Therefore, it should be explored in detail if immunodominant epitopes are indeed the preferred choice for vaccine candidates 272.
Finally, it may be important to identify ways to induce protective immunity specifically in the lungs. Targeting lung tissues directly may require alternative approaches to application, such as delivery using inhalers or nebulisers270.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
The pathogenesis and progression of TB are complex 273. Infection varies by lesion location and the manifestations of pulmonary and extra-pulmonary infection (such as bone, lymphatic, enterophthisis, and meningeal) differ significantly. The progression of TB also presents some challenges to designing pre-clinical research programmes; M. tuberculosis infection can exist as latent TB or manifest as active TB, including primary TB, blood disseminated TB, and secondary TB. Although a wide variety of animal models exist, each can only mimic one or several aspects of TB, but not all forms. To understand the complete picture of human TB, all features of TB need to be replicated in various TB models for different research purposes. Simply put, to quote one expert, “animal models for TB are lousy” 28.
Reliable correlates of human protection or biomarkers for TB have not yet been identified, further limiting pre-clinical research. Monofunctional (IFN-γ secreting) and multifunctional (secreting IFN-γ, TNF, IL-2) CD4+ T cells are currently used as markers for immune protection, but accumulating experimental evidence suggests that host resistance against M. tuberculosis is independent of IFN- γ and TNF secretion from CD4+ T cells. An expert explains that “a major issue is the lack of correlates of protection. There is some insight from epidemiology observations but the basic science is still lacking” 28.
The lack of information regarding correlates of protection or biomarkers limits clinical as well as pre-clinical research. Clinical R&D of vaccine candidates also faces difficulties, as there is currently no safe human challenge model for M. tuberculosis. A recent study demonstrated the feasibility of intradermal challenge with BCG (not virulent M. tuberculosis) but results remain to be validated in field efficacy trials 274.
Clinical trial infrastructure also presents barriers to the development of clinical programmes. Facilities to conduct trials are often not available, and when available, often lack sufficient infrastructure or experience. It is therefore necessary for clinical researchers to collaborate with established networks with proven trial capacity, such as networks for HIV and malaria 275, or existing networks for treating comorbid infectious diseases. One expert explains a successful approach will “utilise the extensive infrastructure that already exists to treat HIV/TB coinfection” 28.
Simple diagnostics for surveillance are currently not available. Little information has been gained thus far about the functional roles of markers related to protection from natural infection, and even less is known about markers of protection from vaccines 276. Recently, however, a study based on transcriptomic profiles from M. tuberculosis -exposed individuals revealed that progression toward active TB can be detected up to 12 months prior to onset of active disease 276. This finding could prove helpful for trial design and allow researchers to reduce study size and duration by focusing on high-risk individuals.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as fairly low (score of 0.5 out of 2).
Expected policy stance
Policy makers strongly endorse BCG and are therefore likely to endorse a broadly protective M. tuberculosis vaccine, too. Almost 2 billion people worldwide have TB 271, and the WHO recommends BCG in settings with high incidence of TB. Experts explain that policy maker support for TB vaccines arises from the disease burden, stating “the argument for development is driven by the number of deaths, the vast majority of which are [caused by] multi-drug resistant [M. tuberculosis]” 28.
Scoring: Based on the above analysis, the expected policy stance was categorised as high (score of 2 out of 2).
Payer, government, or Gavi support
Support for a M. tuberculosis vaccine is likely for countries at all income levels. In high-income countries, BCG currently has support in high-risk geographies (for example, in urban areas), but cost-effectiveness does not meet requirements for routine vaccination 277. Cost-effectiveness considerations would most likely be revised and considered more positively if a broadly and highly efficacious M. tuberculosis vaccine were available. In middle-income countries, a new vaccine would likely replace BCG using the same strategy already in place; depending on cost-effectiveness, vaccination programmes could be expanded in these regions. Finally, Gavi does not support BCG; however, given the high prevalence, mortality, and morbidity caused by TB, a more efficacious vaccine would likely meet Gavi criteria for investment.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as high (score of 2 out of 2).
Barriers to uptake
Few substantial barriers exist to uptake of a novel efficacious M. tuberculosis vaccine replacing BCG. No new touchpoint needs to be developed; the BCG vaccine is already part of childhood vaccination programmes and offered either routinely or in at-risk areas, depending on a country’s disease burden. Where routine vaccination is in place, coverage is already at approximately 90% 264. There are no issues of cultural acceptability to a M. tuberculosis vaccine and changes to clinical practices would not be required; BCG is already part of vaccine recommendations and any more efficacious vaccine would likely replace BCG in existing programmes and recommendations.
Scoring: Based on the above analysis, barriers to uptake was categorised as low (score of 2 out of 2).
Commercial attractiveness
Overall, a new M. tuberculosis vaccine will be commercially attractive given the high expected uptake in low- and middle-income countries where the disease burden is highest. Moreover, if a new vaccine proves to be more efficacious than the existing BCG vaccine, uptake in high-income countries would most likely increase. Some high-income countries currently do not recommend BCG as a routine vaccine because of its low efficacy and the low disease burden in high-income regions, but a more efficacious vaccine could prompt high-income countries to consider broader adoption.
Scoring: Based on the above analysis, commercial attractiveness was categorised as high (score of 2 out of 2).
Primary recommendation
Improving translatability of pre-clinical research would accelerate development of an effective M. tuberculosis vaccine. This can be achieved in several ways including developing animal models with improved predictive capacity; improving and establishing human controlled infection protocols to facilitate early clinical trials; establishing reliable correlates of protection; and testing novel approaches earlier in human trials.
Testing novel approaches earlier in human trials would likely have multiple benefits. Existing animal models are still poor predictors of effectiveness in humans. This creates the risk of investing time and research efforts in candidates that appear promising at the pre-clinical level but ultimately generate disappointing results in clinical trials. Testing earlier in human trials would circumvent this issue, especially as human controlled infection models are becoming available that may greatly reduce the cost and duration of well-powered field studies.
Secondary recommendation
Expanding pre-clinical research to better understand M. tuberculosis infection and host responses is needed to provide critical insights to development of an effective vaccine. Furthermore, investments in novel vaccine technologies that amplify and direct the immune response would likely facilitate vaccine development. Expanding research would require broadening the investment funding envelope – experts state that TB “research is underfunded” 28 and compare the current status of TB funding to other disease states, noting “When HIV was killing less people, it was attracting 1 billion per year. Funders often undervalue the TB effort” 28. In fact, according to the G-FINDER public search tool provided by Policy Cures Research, almost 7 times more funding was provided for the development of preventative HIV vaccines compared to TB vaccines from 2012-2016 278.