Salmonella Typhi
Salmonella Typhi (S. Typhi) causes typhoid fever, a systemic infectious disease characterised by symptoms of fever and abdominal pain. There are ~12 million cases and ~130,000 deaths annually. Previously available vaccines had limitations in their capacity to induce protective immunity. A new, conjugated S. Typhi vaccine has recently been pre-qualified by the WHO and is supported by Gavi for introduction in 2019, following effectiveness trials.
S Typhi falls into a cluster of pathogens for which the priority is to increase vaccine uptake. The primary recommendation is to drive coverage and equity. The secondary recommendation is to incentivise the development of multi-pathogen/combination vaccines.
Salmonella are Gram-negative bacteria of the family Enterobacteriaceae but listed separately on the WHO priority pathogen list. Typhoid fever is an enteric fever caused by Salmonella enterica serovar Typhi (S. Typhi). Typhoid fever is a systemic febrile infection that occurs only in humans 352 and is different from the more common self-limited acute gastroenteritis caused by other Salmonella serotypes 342. Typhoid fever is characterised by high fever, lassitude, abdominal pain, headache, loss of appetite and nausea, dry cough, and occasionally a rash of flat, rose-coloured spots at peak of fever after 7-10 days 353.
Geographically, S. Typhi infection is concentrated in South and South East Asia, and sub-Saharan Africa with many island nations of Oceania also experiencing high incidences and large outbreaks 352. It is less common in industrialised regions such as the United States, Canada, western Europe, Australia, and Japan 354. Transmission is primarily by the faeco-oral route but in rare cases can be transmitted directly from person to person through food handling 334,335. Groups at highest risk for S. Typhi include individuals with immunosuppressive illnesses such as AIDS 345 and those with gastric achlorhydria, which increases susceptibility to S. Typhi infection 344. According to the IHME, there are an estimated 12 million cases of typhoid fever per year globally 31.
Direct health impact
The IHME gathers data on the burden of S. Typhi infection. This data source has a defined methodology and is used and accepted in the global health community. The IHME estimates that S. Typhi causes 130,000 deaths per year and 113,000 years lived with disability per year 31.
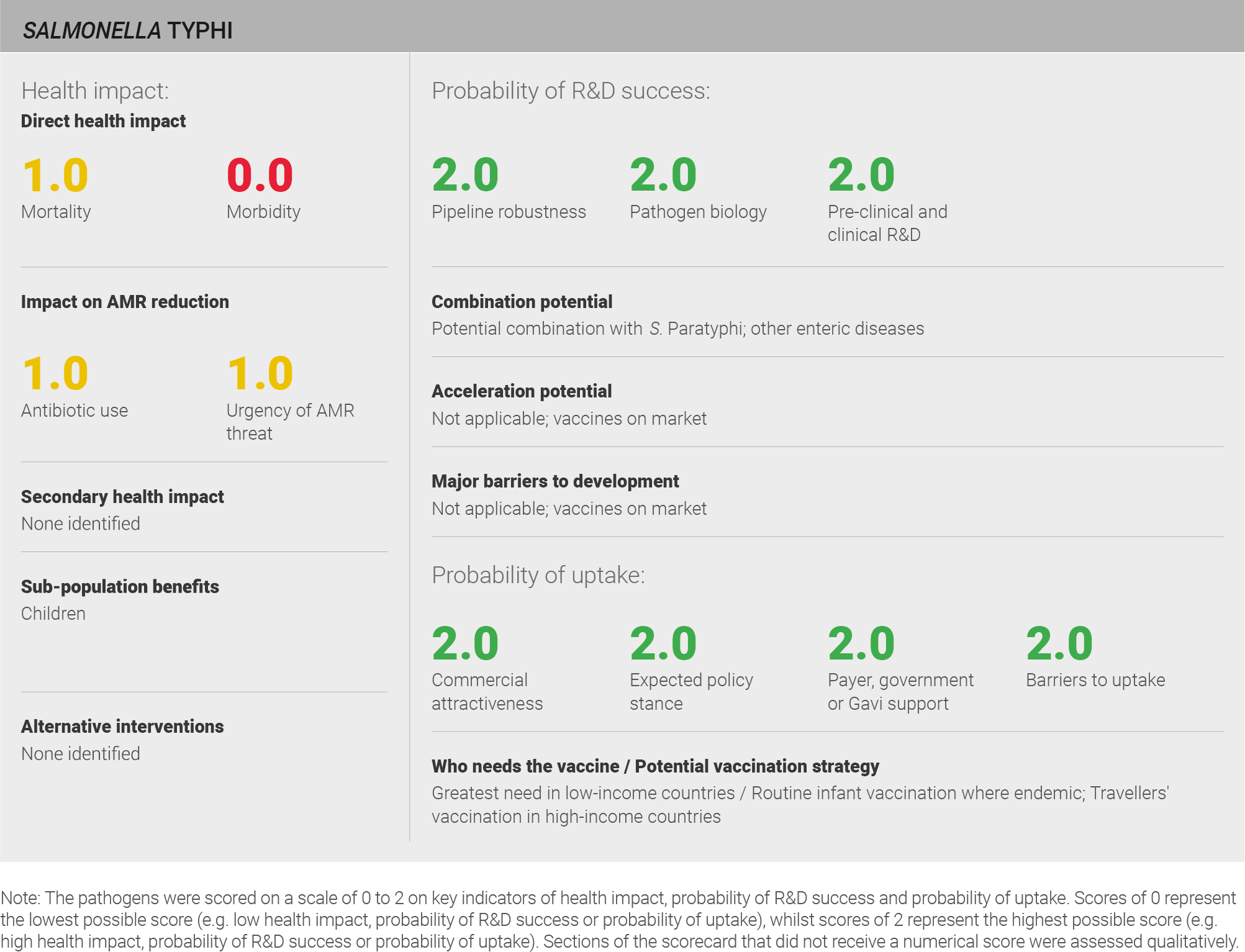
Scoring: Based on the above analysis, mortality was characterised as medium (score of 1 out of 2) and morbidity was characterised as low (score of 0 out of 2).
Sub-population benefits
Infants and young children, and patients with conditions that predispose them to infection (such as immunosuppressive illnesses including AIDS 345 and gastric achlorhydria, which increases susceptibility to S. Typhi infection 344) are most likely to benefit from vaccination.
Antibiotic use
S. Typhi infections are primarily treated with fluoroquinolones, third-generation cephalosporins, and azithromycin. Carbapenems are reserved for suspected infection with extensively drug-resistant strains 346. Many experts consider fluoroquinolones such as ciprofloxacin or ofloxacin to be the drug of choice for susceptible isolates 28. When fluoroquinolones cannot be used, azithromycin or cephalosporins are alternatives.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~12 million typhoid cases and treated with a two week course of antibiotics.
Urgency of AMR threat
WHO has listed Salmonella spp. as ‘high’ in its priority list of R&D for new antibiotics and the CDC lists it as a ‘serious’ threat in its list of biggest threats from AMR 7.
Multi-drug resistant strains to ampicillin, trimethoprim-sulfamethoxazole and chloramphenicol are widespread globally preventing these agents being used to treat S. Typhi 346. The presence of multi-drug resistant strains varies widely from 10-80% globally 346.. Most strains remain susceptible to azithromycin and ceftriaxone but an outbreak of extensively drug-resistant typhoid with resistance to ampicillin, trimethoprim-sufamethoxazole, chloramphenicol, fluoroquinolones, and ceftriazone was recently reported355.
Scoring: Based on the above analysis, the urgency of AMR threat was characterised as medium (score of 1 out of 2).
Pipeline robustness
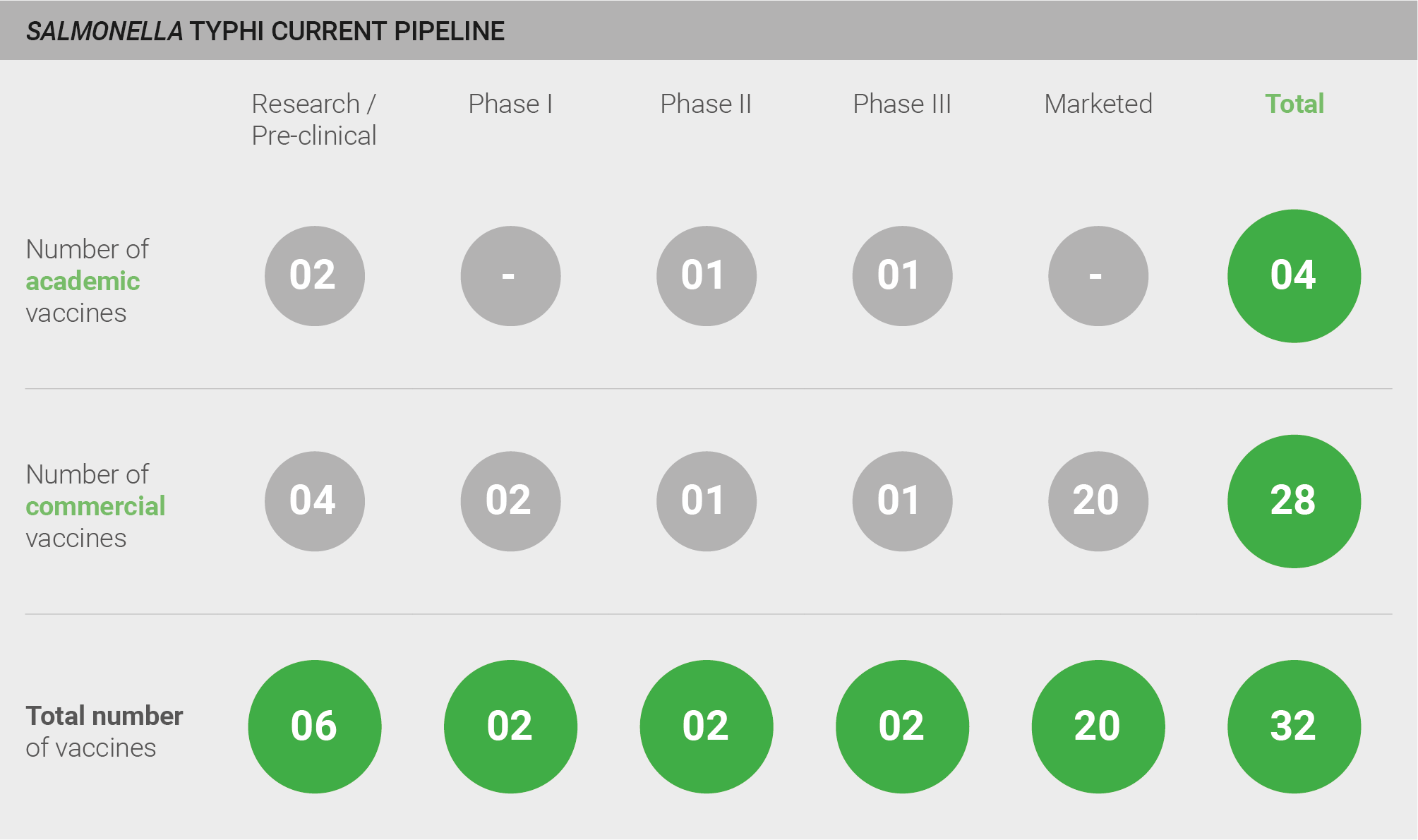
The pipeline for development of vaccines against S. Typhi comprises a total of 20 marketed vaccines and 12 vaccines currently in development. Six vaccines are in pre-clinical development, two are in Phase I, two are in Phase II, and two are in Phase III.
Marketed vaccines fall into two groups: live attenuated Ty21a oral vaccines and Vi polysaccharide vaccines. The live attenuated Ty21a oral vaccine was developed in the early 1970s by chemical-induced mutagenesis of a pathogenic S. Typhi strain that no longer expressed the Vi polysaccharide 352. The limitation of this vaccine is that the level of protective immunity varies widely depending on the vaccine formulation, number of doses administered, and interval between doses. The Vi polysaccharide vaccine comprises a highly purified Vi polysaccharide. However, unconjugated polysaccharide vaccines – including this vaccine – are limited by poor immunogenicity in infants and young children and by short-lived duration of protection 352.
To overcome these limitations novel strategies have been pursued. Recently two newer generation Typhoid conjugate vaccines (TCV) were licensed. Typbar-TCV®, and PedaTyph™ 352. Both TCV consist of the Vi polysaccharide conjugated to a protein carrier 352 that enhances immunogenicity and converts the short-lasting T-cell independent Vi-specific immune response to a T-cell dependent response which is preferred given the induction of antibodies with higher affinity and also of long-lasting memory 348. Experts explain that this modification is critical to improving on previous vaccines, with one expert explaining “conjugation is very important because that is what drives immunity in children” 28. This approach has shown safety and efficacy in field trials, as well as efficacy in controlled human infection studies 356 and one expert anticipates “I expect effectiveness in the field to be even higher” 28.
Typbar TCV® is currently in effectiveness trial studies in several countries 357 as experts note they are “looking for effectiveness in Bangladesh, Nepal, India, Malawi and outbreak response in Pakistan” 28. Other TCV candidates are in clinical development or already undergoing licensure review 352.
Scoring: Based on the above analysis, pipeline robustness was categorised as high (score of 2 out of 2).
Current pipeline
Pathogen biology
Although repeat clinical episodes of S. Typhi infection have been described, they are uncommon, suggesting that immune responses are mostly protective following initial episodes of infection 352.
Immunological protection against typhoid fever is believed to involve both cell-mediated and humoral responses given the fact that Salmonellae are facultative intracellular pathogens and exist both extracellularly and within intracellular niches (specifically monocytes and macrophages). Following natural infection, specific antibodies are detected in both serum and in the intestines.
Scoring: Based on the above analysis, pathogen biology was categorised as high (score of 2 out of 2).
Pre-clinical and clinical R&D
Many, varied animal models are in use for pre-clinical research on S. Typhi vaccine candidates 350.
Mouse models have been the most utilised. However, as S. Typhi is human host restricted and normally asymptomatic in mice, a lethal infection has to be produced in mice by suspending the bacteria in hog gastric mucin and then injecting the suspension intraperitoneally. Results from other models including larger animal models, such as non-human primates, may provide a more complete assessment of vaccine candidates and should be prioritised for vaccine candidates already evaluated in small animal models 350.
WHO deems estimation of total anti-Vi IgG to be an appropriate measure of vaccine immunogenicity for S. Typhi, and suggests evaluation of new conjugate vaccines against Vi polysaccharide in immunogenicity trials 358, providing a quasi-correlate of protection that facilitates both pre-clinical and clinical research.
Controlled human infection models exist and have been part of established pathways to licensure 14,17 and sufficient clinical trial infrastructure exists in endemic regions 359. Finally, there is an established route to approval for S. Typhi vaccines.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as high (score of 2 out of 2).
Expected policy stance
The typhoid conjugate vaccine is particularly helpful for children in endemic settings. A protective vaccine would therefore likely be administered to all children in endemic regions. Furthermore, a potential vaccine would be useful for travellers.
Policy support for S. Typhi vaccination is strong as S. Typhi has a high incidence of ~12 million cases per year, with high mortality 31. Even before the approval of TCV, the need for improved vaccines was recognised by both experts and policy makers, with one expert noting “[S. Typhi was] a prime candidate for WHO support. We have vaccines against typhoid, but with short duration of protection” 28. WHO recommends a single dose of TCV at six-nine months of age in endemic settings, and studies are examining co-administration possibilities at nine months 28.
Scoring: Based on the above analysis, expected policy stance was categorised as high (score of 2 out of 2).
Payer, government or Gavi support
A Typhoid vaccine is already recommended for travellers by UK and American agencies 360,361, and improved vaccines are likely to continue to receive support for travellers in high-income countries.
In middle-income countries, the high disease burden makes support likely in areas where S. Typhi infection is endemic. Support is also likely in low-income countries as in May 2018 Gavi earmarked $85 million for a prequalified TCV 362.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as high (score of 2 out of 2).
Barriers to uptake
Few barriers exist for a S. Typhi vaccination programme. Vaccination against S. Typhi is already part of childhood vaccination programmes and offered to travellers, so no new touchpoints or changes to existing clinical practices would be required.
Scoring: Based on the above analysis, barriers to uptake were categorised as low (score of 2 out of 2).
Commercial attractiveness
Commercial attractiveness is high in view of the existence of recently licenced commercial vaccines, Gavi’s stated interest in and support for vaccination against S. Typhi in low-income countries, likely uptake in middle-income countries where infection is endemic, and the travellers´ vaccine market in high-income countries.
Scoring: Based on the above analysis, commercial attractiveness was categorised as high (score of 2 out of 2).
Primary recommendation
The primary recommendation is to drive coverage and equity. Current coverage with TCV is low given its recent licensure and ongoing effectiveness trials. Other vaccine candidates in development by alternative producers should also be supported to ensure healthy competition and production capacity in the market.
Secondary recommendation
The secondary recommendation is to incentivise multi-pathogen/combination vaccines, including combination vaccines with S. Paratyphi and with non-invasive enteric strains given the strong policy interest in enteric vaccine combinations.