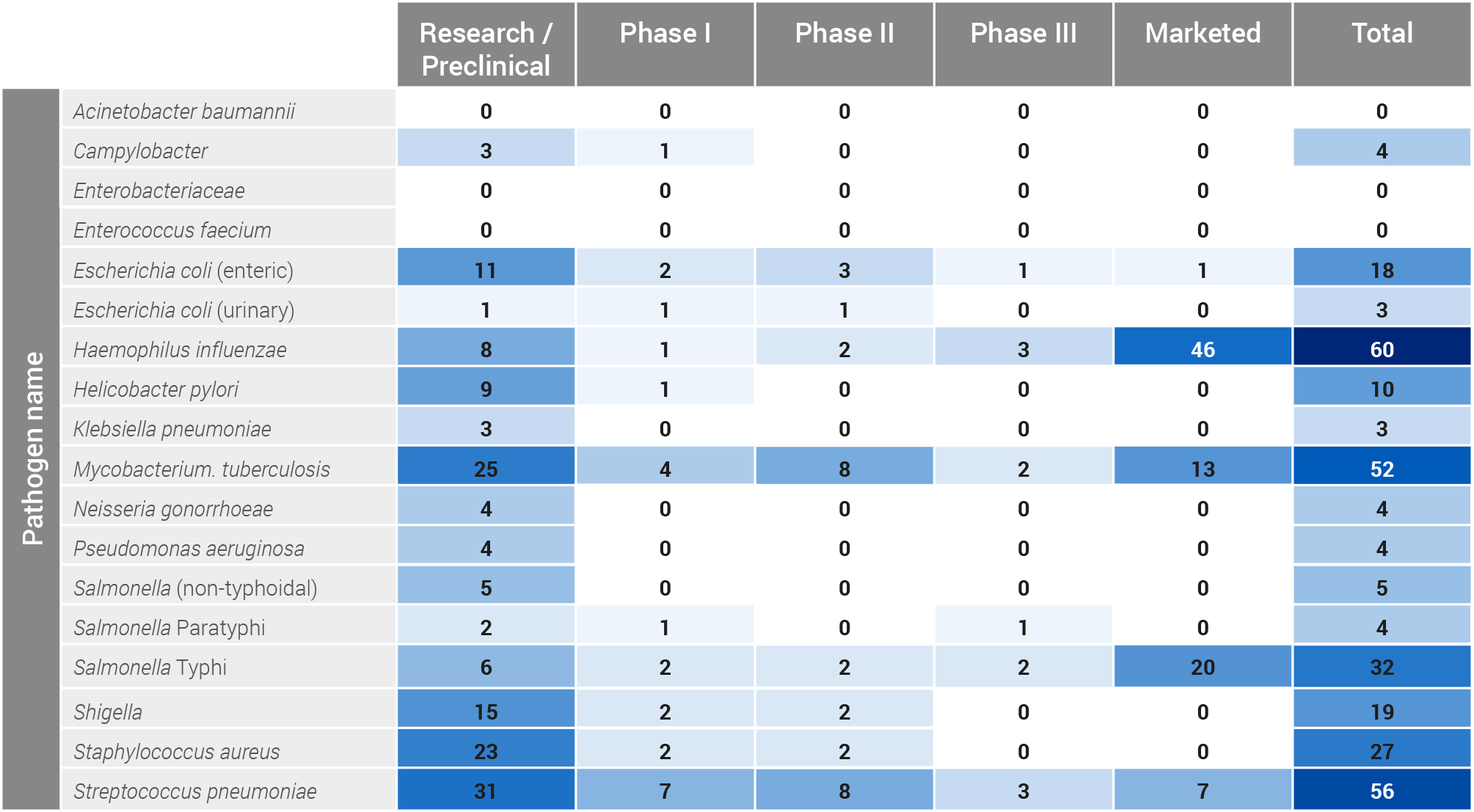
| Campylobacter
|
Campylobactor/ETEC Vaccines
|
Immuron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Campylobacter
|
Campylobacter Research Program
|
Vir Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Campylobacter
|
Campylobacter jejuni capsule conjugate
|
US Department of Defense
|
Academic
|
Phase I
|
Riddle et al., 2016, Vaccine
|
| Campylobacter
|
PEB1 DNA prime/protein boost
|
Shandong Medical College
|
Academic
|
Research / Preclinical
|
Riddle et al., 2016, Vaccine
|
| Escherichia coli (enteric)
|
Dukoral
|
Johnson & Johnson |
Commercial
|
Marketed
|
PharmaProjects
|
| Escherichia coli (enteric)
|
ETEC & Shigella vaccine |
University of Maryland
|
Academic
|
Phase I
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
Etvax
|
Scandinavian Biopharma
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
ETEC vaccine
|
Eubiologics
|
Commercial
|
Phase III
|
PharmaProjects
|
| Escherichia coli (enteric)
|
E. coli (LT) Vaccine
|
U.S. Army Medical
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
Bacterial diarrhoea vaccine
|
Prokarium
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
E. coli Vaccine Program
|
Syntiron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
ETEC vaccine
|
Hilleman Laboratories
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Escherichia coli (enteric)
|
E. coli vaccine
|
Mucosis
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Escherichia coli (enteric)
|
CDX-EC
CDXEC
|
Codagenix
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Escherichia coli (enteric)
|
ETEC/Cholera vaccine, Valneva; VLA 1701; VLA-1701; VLA1701
|
Valneva
|
Commercial
|
Phase II
|
PharmaProjects
|
| Escherichia coli (enteric)
|
Campylobactor/ETEC Vaccines
|
Immuron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Escherichia coli (enteric)
|
ACE527
|
PATH, NVSI, UGA
|
Academic
|
Phase II
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
| Escherichia coli (enteric)
|
FTA
|
PATH, NMRC, Sanofi, IDRI
|
Commercial
|
Phase I
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
| Escherichia coli (enteric)
|
Ty21a typhoid vaccine expressing Shigella LPS and MEFA
|
Protein Potential LLC.
|
Commercial
|
Research / Preclinical
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
| Escherichia coli (enteric)
|
CVD GuaBA mutants expressing ETEC antigens
|
UMB, PaxVax
|
Commercial
|
Research / Preclinical
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
| Escherichia coli (enteric)
|
MEFA
|
KSU, JHU, PATH
|
Academic
|
Research / Preclinical
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
| Escherichia coli (enteric)
|
LT/ST Fusion/conjugate
|
ENTVAC Consortium, PATH
|
Academic
|
Research / Preclinical
|
PATH - Status of Vaccine Development for ETEC (27 June 2018)
|
|
E. coli (urinary)
|
ExPEC Vaccine
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| E. coli (urinary)
|
UPEC Vaccine Program
|
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| E. coli (urinary)
|
UTI Vaccine Program
|
Sequoia Sciences
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Haemophilus influenzae
|
Vaxelis
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Eupenta
|
LG Chem
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Haemophilus influenzae
|
Actacel
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
ActHIB
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Bactolisato
|
Grupo De Laboratorios Leti
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Biohib
|
Bharat Biotech
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
ComBEfive
|
Biological E
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Comvax
|
Merck & Co |
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
DTaP-IPV/Act-Hib
|
Statens Serum Institut
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Easyfive-TT
|
Panacea Biotec
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Easyfour-TT
|
Panacea Biotec
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Easysix-TT
|
Panacea Biotec
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Euforvac-Hib
|
LG Chem
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Euhib
|
LG Chem
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Haemophilus influenzae Type b Conjugate Vaccine
|
Walvax Biotechnology
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Haemophilus influenzae Type b Conjugate Vaccine
|
Biological E
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Haemophilus influenzae Type b Vaccine
|
Chongqing Zhifei Biological Products
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Hexacima
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Hib conjugate vaccine
|
Sun Pharmaceutical Industries
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Hib Vaccine
|
PT Bio Farma
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
HibACon
|
Chongqing Zhifei Biological Products
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Hiberix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
HibTITER
|
Pfizer
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Infanrix Hep B-IPV/Hib
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Infanrix Hib
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Infanrix IPV/Hib
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
MenHibrix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Menitorix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
NovoHib
|
Panacea Biotec
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pediacel
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pedvax HIB
|
Merck & Co |
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pentabio
|
PT Bio Farma
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pentacel
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
PENTAct-HIB
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pentavac
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Pentaxin
|
Center for Research and Production of Vaccines and Biologicals (POLYVAC)
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Provax
|
Almirall
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Quimi-Hib
|
VABIOTECH
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Quintanrix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Quinvaxem
|
Johnson & Johnson |
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Shan Hib
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Shan4
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Shan5
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Sii HibPRO
|
Serum Institute of India
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
Tetracel
|
Pfizer
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
TriHIBit
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
TritanrixHB
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Haemophilus influenzae
|
LBVD
|
LG Chem
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Haemophilus influenzae
|
Shan 6
|
Sanofi
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Haemophilus influenzae
|
GSK2838497A
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Haemophilus influenzae
|
MT-2355
|
Mitsubishi Tanabe Pharma
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Haemophilus influenzae
|
VN-0105
|
Sanofi
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Haemophilus influenzae
|
DTcP-Hib Combo Vaccine
|
Tianjin CanSino Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
MCV2-Hib Combo Vaccine
|
Tianjin CanSino Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
ACYW135-Hib Polysaccharide Conjugate Vaccine
|
Chongqing Zhifei Biological Products
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
DTP-Hib Vaccine
|
Zydus Cadila
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
Haemophilus influenzae Type b Conjugate Vaccine
|
Wellstat Group
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
Liquid Hexavalent Vaccine
|
Biological E
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
Haemophilus influenza vaccine |
The University of Iowa
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Haemophilus influenzae
|
Liquid Hexavalent Vaccine
|
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Helicobacter pylori
|
IMX101
|
ImevaX
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Helicobacter pylori
|
Helicobacter pylori vaccine |
HeliCure
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Helicobacter pylori
|
H. pylori vaccine |
EpiVax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Helicobacter pylori
|
H. pylori vaccine |
Academy of Military Medical Sciences
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Helicobacter pylori
|
H. pylori vaccine |
ImmunoBiology
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Helicobacter pylori
|
H. pylori vaccine |
ImmBio
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Helicobacter pylori
|
Urease epitope vaccine
|
Sichuan University
|
Academic
|
Research / Preclinical
|
Sutton et al., 2018, Vaccine
|
| Helicobacter pylori
|
Lp220 vaccine
|
Southern Medical University
|
Academic
|
Research / Preclinical
|
Sutton et al., 2018, Vaccine
|
| Helicobacter pylori
|
Probiotic vaccine delivery
|
China Pharmaceutical University
|
Academic
|
Research / Preclinical
|
Sutton et al., 2018, Vaccine
|
| Helicobacter pylori
|
Gastric Cancer Vaccine
|
MCRI (Murdoch Children's Research Institute)
|
Academic
|
Research / Preclinical
|
Sutton et al., 2018, Vaccine
|
| Klebsiella pneumonia
|
Klebsiella pneumoniae Vaccine Program
|
Syntiron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Klebsiella pneumoniae
|
Klebsiella pneumoniae vaccine
|
Emergex Vaccines
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Klebsiella pneumoniae
|
Klebsiella pneumoniae Vaccine Program
|
Astrogenetix
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine
|
Abera Bioscience
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Ad5Ag85A
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase I
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
DAR-901
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
H1-IC31
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
ID93+GLA-SE
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
MTBVAC
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
SSI H56-IC31
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
MVA Vaccine
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
rCMV
|
Aeras Global TB Vaccine Foundation
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Ruti Vaccine
|
Archivel Farma
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Antitubercle Vaccine BCG 10
|
Biomed-Lublin
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Vaccae
|
Chongqing Zhifei Biological Products
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine
|
Chongqing Zhifei Biological Products
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
TB Vaccine
|
EpiVax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis GTU Vaccine Research Project |
FIT Biotech
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
GC3107
|
GC Pharma
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Vaccine
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
GSK M72
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine |
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
GI-19000
|
GlobeImmune
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis Research Project |
GlobeImmune
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Vaccine
|
Green Signal Bio Pharma
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
T-Biovax
|
ImmBio
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
IMM201
|
Immodulon Therapeutics
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Mycobacterium tuberculosis
|
V-5 Immunitor
|
Immunitor
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
V-7 Immunitor
|
Immunitor
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine |
Inovio Pharmaceuticals
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis Vaccine Project |
I'rom Group
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Immuvac
|
Cadila Pharmaceuticals
|
Commercial
|
Marketed
|
PharmaProjects
|
| Mycobacterium tuberculosis
|
Freeze-Dried BCG Vaccine
|
Japan BCG Laboratory
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Freeze-Dried BCG Vaccine
|
Korea Vaccine
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Lipovax-Fg115-TB
|
Lipotek
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Lipovax-FliC-TB
|
Lipotek
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis Research Programme |
Longhorn Vaccines and Diagnostics
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Ad5Ag85A Aerosol
|
McMaster University
|
Academic
|
Phase I
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
VAC B.C.G.
|
medac
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Vaccine
|
PT Bio Farma
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
TB Vaccine
|
Recipharm
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG vaccine
|
Sanofi
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Aeras-404 (H4:IC31)
|
Sanofi
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
VPM1002
|
Serum Institute of India
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Vaccine
|
SINOPHARM
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Vaccine
|
Statens Serum Institut
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine |
The University of Hawai'i System
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis Research Project |
The University of Melbourne
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine |
The University of Wisconsin System
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Therapeutic MDR Tuberculosis Program
|
Theravectys
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
TB Immunotherapy Program
|
Transgene
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
TVI-Tuberculosis-1
|
TVAX Biomedical
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
BCG Aerosol
|
University of Birmingham
|
Academic
|
Phase I
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
MTbuVax
|
Vaxil BioTherapeutics
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Mycobacterium tuberculosis
|
Tuberculosis vaccine, Greffex |
Greffex
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Neisseria gonorrhoeae
|
Gonorrhoea Vaccine Program |
Novavax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Neisseria gonorrhoeae
|
Gonorrhoea Vaccine Program |
Genocea Biosciences
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Neisseria gonorrhoeae
|
Neisserial Vaccine |
The Rockefeller University
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Neisseria gonorrhoeae
|
Gonorrhoea Vaccine |
The University of Iowa
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Pseudomonas aeruginosa
|
Pseudomonas Vaccine |
Wake Forest University
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Pseudomonas aeruginosa
|
Pseudomonas aeruginosa Vaccine Program
|
Syntiron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Pseudomonas aeruginosa
|
Pseudomonas aeruginosa Vaccine Program
|
Astrogenetix
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Pseudomonas aeruginosa
|
Pseudomonas aeruginosa Vaccine Program
|
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Salmonella (non-typhoidal)
|
NTS Vaccine
|
University of Maryland
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Salmonella (non-typhoidal)
|
CVD NTS Project
|
University of Maryland, Bharat Biotech, Wellcome Trust
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Salmonella (non-typhoidal)
|
Bivalent iNTS-GMMA
|
GlaxoSmithKline (NVGH)
|
Commercial
|
Research / Preclinical
|
Tennant at al., 2016, Vaccine
|
| Salmonella (non-typhoidal)
|
Bivalent conjugate (O:1,4[5],12-CRM197 + O:1,9,12-CRM197)
|
GlaxoSmithKline (NVGH)
|
Commercial
|
Research / Preclinical
|
Tennant at al., 2016, Vaccine
|
| Salmonella (non-typhoidal)
|
OmpD
|
University of Birmingham
|
Academic
|
Research / Preclinical
|
Tennant at al., 2016, Vaccine
|
| Salmonella Paratyphi
|
CVD 1902
|
University of Maryland, Bharat Biotech
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Salmonella Paratyphi
|
Paratyphoid A conjugate vaccine
|
Lanzhou Institute
|
Academic
|
Phase III
|
PharmaProjects
|
| Salmonella Paratyphi
|
O:2,12-DT + Vi-DT [International Vaccine Institute]
|
International Vaccine Institute (IVI)
|
Academic
|
Research / Preclinical
|
Martin et al., 2016, Vaccine
|
| Salmonella Paratyphi
|
O:2,12-CRM197 + Vi-CRM197
|
Biological E
|
Commercial
|
Research / Preclinical
|
Martin et al., 2016, Vaccine
|
| Salmonella Typhi
|
Typhetec
|
Prokarium
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Salmonella Typhi
|
Salmonella Vaccine Project
|
Affinivax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Salmonella Typhi
|
Ttyphoid vaccine
|
Microgen
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
CVD 909
|
University of Maryland
|
Academic
|
Phase II
|
EvaluatePharma
|
| Salmonella Typhi
|
Vi polysaccharide typhoid vaccine
|
China National Pharmaceutical (Beijing Tiantan Biological)
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Tyvax VI plus
|
VHB Life Sciences
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Ttyphoid vaccine
|
Zydus Cadila (Zydus Vaccicare)
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Tyrix Vi
|
SK Holdings
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Shantyph
|
Sanofi (Shantha Biotechnics)
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typhim Vi, Typhyvax
|
Sanofi (Pasteur Mérieux)
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typhobox, Typhovax
|
Green Cross
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typherix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Zerotyph
|
Boryung
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Peda-Typh
|
Bio-Med
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Neotyf, Typhoral, Vivotif
|
Johnson & Johnson |
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
vax-TyVi
|
Finlay Institute
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typhoid-Kovax
|
Sanofi
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typbar-TCV
|
Bharat Biotech
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Hepatyrix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
VIVAXIM
|
Sanofi
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Biovac Typhoid
|
Wockhardt
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
Typho-Vi
|
Bio-Med
|
Commercial
|
Marketed
|
PharmaProjects
|
| Salmonella Typhi
|
enteric fever vaccine
|
Prokarium
|
Commercial
|
Phase II
|
PharmaProjects
|
| Salmonella Typhi
|
Vi-DT typhoid conjugate vaccine
|
Bio Farma
|
Commercial
|
Phase I
|
PharmaProjects
|
| Salmonella Typhi
|
Salmonella typhi + paratyphi vaccine
|
Prokarium
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Salmonella Typhi
|
Vi-rEPA
|
National Health Institute
|
Academic
|
Phase III
|
MacLennan et al., 2014, Human Vaccines & Immunotherapeutics |
| Salmonella Typhi
|
Vi-rEPA
|
Lanzhou Institute (China)
|
Commercial
|
Marketed
|
MacLennan et al., 2014, Human Vaccines & Immunotherapeutics |
| Salmonella Typhi
|
Vi-CRM
|
Biological E
|
Commercial
|
Phase III
|
MacLennan et al., 2014, Human Vaccines & Immunotherapeutics |
| Salmonella Typhi
|
Vi conjugated to fusion protein PsaA-PdT
|
Harvard Medical School
|
Academic
|
Research / Preclinical
|
MacLennan et al., 2014, Human Vaccines & Immunotherapeutics |
| Salmonella Typhi
|
Ty21a typhoid vaccine expressing Shigella LPS
|
Protein Potential LLC.
|
Commercial
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Salmonella Typhi
|
O:2,12-DT + Vi-DT [International Vaccine Institute]
|
International Vaccine Institute (IVI)
|
Academic
|
Research / Preclinical
|
Martin et al., 2016, Vaccine
|
| Salmonella Typhi
|
O:2,12-CRM197 + Vi-CRM197
|
Biological E
|
Commercial
|
Research / Preclinical
|
Martin et al., 2016, Vaccine
|
| Shigella
|
SF2a-TT15 vaccine
|
Institut Pasteur
|
Academic
|
Phase I
|
EvaluatePharma
|
| Shigella
|
ETEC & Shigella vaccine |
University of Maryland
|
Academic
|
Phase I
|
EvaluatePharma
|
| Shigella
|
Flexyn2a
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Shigella
|
1790GAHB vaccine
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Shigella
|
Bacterial diarrhoea vaccine
|
Prokarium
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Shigella
|
Shigella Vaccine Program
|
Immuron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Shigella
|
Multivalent Shigella Vaccine
|
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Shigella
|
Shigella vaccine
|
Merck & Co. |
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Shigella
|
Shigella vaccine
|
Immuron
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Shigella
|
Shigella vaccine
|
Chongqing Zhifei Biological
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Shigella
|
Quadrivalent Shigella Vaccine
|
University of Maryland
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Shigella
|
Holotoxoid Vaccine
|
Uniformed Services University of the Health Sciences
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Shigella
|
CVD 1208, CVD 1213 and CVD 1215
|
University of Maryland
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
Truncated Shigella
|
International Vaccine Institute, Seoul, Korea
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
Ty21a typhoid vaccine expressing Shigella LPS
|
Protein Potential LLC.
|
Commercial
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
Inactivated trivalent Shigella whole cell
|
PATH, Washington DC and WRAIR, Silver Spring, Maryland USA
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
Heat Killed Multi Serotype Shigella (HKMS) vaccine
|
NICED, Kolkata, India
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
DB Fusion
|
PATH
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Shigella
|
34 kDa OmpA
|
NICED, Kolkata, India
|
Academic
|
Research / Preclinical
|
Mani et al., 2016, Vaccine
|
| Staphylococcus aureus
|
STEBVax
|
Integrated BioTherapeutics
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staph Aureus Vaccine |
GlaxoSmithKline
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Staphylococcus aureus
|
PF-06290510
|
Pfizer
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Staphylococcus aureus
|
NDV-3A
|
NovaDigm Therapeutics
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Staphylococcus aureus
|
MRSA Vaccine
|
Sanofi
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
MVA-BN MRSA Vaccine
|
Bavarian Nordic
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
IBT-V02
|
Integrated BioTherapeutics
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Vaccine
|
Syntiron
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
S. aureus vaccine research program |
Absynth Biologics
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Bellerophon Project
|
IMAXIO
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Methicillin-resistant Staphylococcus aureus (MRSA) Research project
|
Bharat Biotech
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Superantigen Toxin Vaccine
|
U.S. Army Medical
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Vaccine
|
U.S. Army Medical
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Vaccine
|
University of Minnesota
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
MRSA Vaccine Program
|
University of Maryland
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Serenta-University of Maryland Research Project
|
University of Maryland
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
MRSA Vaccine Project
|
VLP Biotech
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Bioconjugate Vaccine Program
|
GlaxoSmithKline
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Bacterial Vaccine Research Program
|
Ludwig Maximilians University
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus vaccine
|
Abcombi Biosciences
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Staphylococcus aureus
|
Methicillin-resistant Staphylococcus Aureus (MRSA) Research project
|
SBI Holdings
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Vaccine Program
|
Astrogenetix
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcal Vaccine
|
Uniformed Services University of the Health Sciences
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
Staphylococcus Aureus Vaccine Project
|
Affinivax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Staphylococcus aureus
|
AV-0328; AV0328
|
Alopexx Vaccine, LLC
|
Commercial
|
Phase I
|
PharmaProjects
|
| Staphylococcus aureus
|
Staphylococcus Aureus infection therapy, Immunartes
|
ImmunArtes
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Staphylococcus aureus
|
Staphylococcus Aureus vaccine, Astellas
|
Astellas Pharma
|
Commercial
|
Research / Preclinical
|
PharmaProjects
|
| Staphylococcus aureus
|
Staphylococcus Aureus ghost
|
Pan Chai University
|
Academic
|
Research / Preclinical
|
Giersing et al., 2016, Vaccine
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
Abera Bioscience
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
Affinivax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Streptococcus Pneumoniae Research Project |
Affinivax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
Astellas Pharma
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Streptococcus pneumonia Vaccine Program |
Astrogenetix
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
15 Valent Pneumococcal conjugate Vaccine
|
Aurobindo Pharma
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Conjugate Vaccine
|
Biological E
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Hib Vaccine
|
BioNet-Asia
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
13-Valent Pneumococcal conjugate Vaccine
|
Chongqing Zhifei Biological Products
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
23-Valent Pneumococcal conjugate Vaccine
|
Chongqing Zhifei Biological Products
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
Eurocine Vaccines
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
Gamma Vaccines
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
GSK2189242A
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
S. Pneumoniae Vaccine Program
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
NTHi-Pneumo
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
GSK2830929A
|
GlaxoSmithKline
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Synflorix
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumopur/Steptopur
|
GlaxoSmithKline
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
PnuBiovax
|
ImmBio
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine Program
|
Instituto Butantan
|
Academic
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Streptococcus Pneumoniae Vaccine |
Integrated BioTherapeutics
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
LBVE
|
LG Chem
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Multivalent Pneumococcal Vaccine
|
Liquidia Technologies
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
V114
|
Merck & Co |
Commercial
|
Phase III
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumovax
|
Merck & Co |
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Osaka University-BIKEN Pneumococcal Vaccine
|
Osaka University
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Nucovac
|
Panacea Biotec
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumo Nexgen Vaccine
|
Pfizer
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
PF-06482077
|
Pfizer
|
Commercial
|
Phase II
|
EvaluatePharma
|
| Streptococcus pneumoniae |
PF-06842433
|
Pfizer
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Prevnar 13
|
Pfizer
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Prevnar
|
Pfizer
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine TruePatch
|
Prometheon Pharma
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Conjugate Vaccine
|
Sanofi
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Streptococcus Pneumoniae Vaccine
|
Sanofi
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Polysaccharide Conjugate Vaccine
|
Serum Institute of India
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Huiyikang
|
SINOPHARM
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal 13-valent Conjugate Vaccine (PCV)
|
Sinovac Biotech
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal 23-valent Polysaccharide Vaccine (PPV)
|
Sinovac Biotech
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Streptococcus pneumoniae |
NBP606
|
SK Chemicals
|
Commercial
|
Phase III
|
EvaluatePharma
|
| Streptococcus pneumoniae |
CBPG Protein
|
St. Jude Children's Research Hospital
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Streptococcus Pneumoniae Vaccine |
St. Jude Children's Research Hospital
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Conjugate Vaccine
|
SutroVax
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine Program
|
Synergy America
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
15 Valent Pneumococcal conjugate Vaccine
|
Tergene Biotech
|
Commercial
|
Phase I
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Osaka University-BIKEN Pneumococcal Vaccine
|
The Research Foundation for Microbial Diseases of Osaka University (BIKEN)
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
GlpO
|
The University of Adelaide
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Immunoregulatory Therapy
|
The University of Newcastle
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine
|
The University of Pennsylvania
|
Academic
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal 13-valent Conjugate Vaccine (PCV)
|
Tianjin CanSino Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Protein Vaccine
|
Tianjin CanSino Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Polysaccharide Vaccine
|
Tianjin CanSino Biotechnology
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Anti-Pneumococcal Vaccine
|
Vaxxilon
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcal Vaccine Program
|
Virometix
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|
| Streptococcus pneumoniae |
PPV23
|
Walvax Biotechnology
|
Commercial
|
Marketed
|
EvaluatePharma
|
| Streptococcus pneumoniae |
Pneumococcus Conjugate Vaccine
|
Wellstat Group
|
Commercial
|
Research / Preclinical
|
EvaluatePharma
|