Staphylococcus aureus
Staphylococcus aureus (S. aureus) is a major cause of skin infections and, when invasive, can also cause more serious conditions, including endocarditis and pneumonia. S. aureus accounts for ~10% of pneumonia cases and ~30% of cellulitis cases worldwide. AMR is a serious concern. Methicillin-resistant Staphylococcus aureus (MRSA) is widespread and ~50% of all staphylococcus infections are now methicillin resistant. Whilst vaccine development remains challenging with several recent failures, there is strong interest from industry and four candidates are in clinical development. The initial target population for a vaccine is likely to be elective surgery patients. A vaccine would likely see reasonable uptake in high- and middle-income countries because the high economic burden of infection would drive a favourable cost-effectiveness assessment. However, uptake in low-income countries would likely require novel financing mechanisms.
S. aureus falls into a cluster of pathogens for which advancing early R&D is the priority. The primary recommendation is to support pre-clinical research. The secondary recommendations are to explore alternative treatment and prevention strategies and to better understand the burden, epidemiology and transmission.
S. aureus is a Gram-positive commensal bacterium that is associated with both community- and hospital-acquired infections. S. aureus is commonly found on the skin or in the nasopharynx and can be transmitted through skin-to-skin contact 381. The most common manifestations of S. aureus infection are cellulitis and lower respiratory tract infection, but it can affect a variety of organs and tissues, causing endocarditis, osteomyelitis, and septic arthritis 381,382. .
Symptoms vary depending on the site of infection. Cellulitis typically manifests as local warmth, erythema, pain, and fever, while lower respiratory tract infection is associated with productive cough, shortness of breath, fever, tachypnoea, and reduced oxygen saturation.
Groups at high risk for S. aureus infection include populations with weakened immune systems, people with chronic conditions (including diabetes, cancer, HIV, vascular disease, eczema and lung disease), surgical patients, and both very young and elderly populations.
S. aureus infection has a global distribution, but there are some gaps in understanding of the specific disease epidemiology in low- and middle-income countries 383.
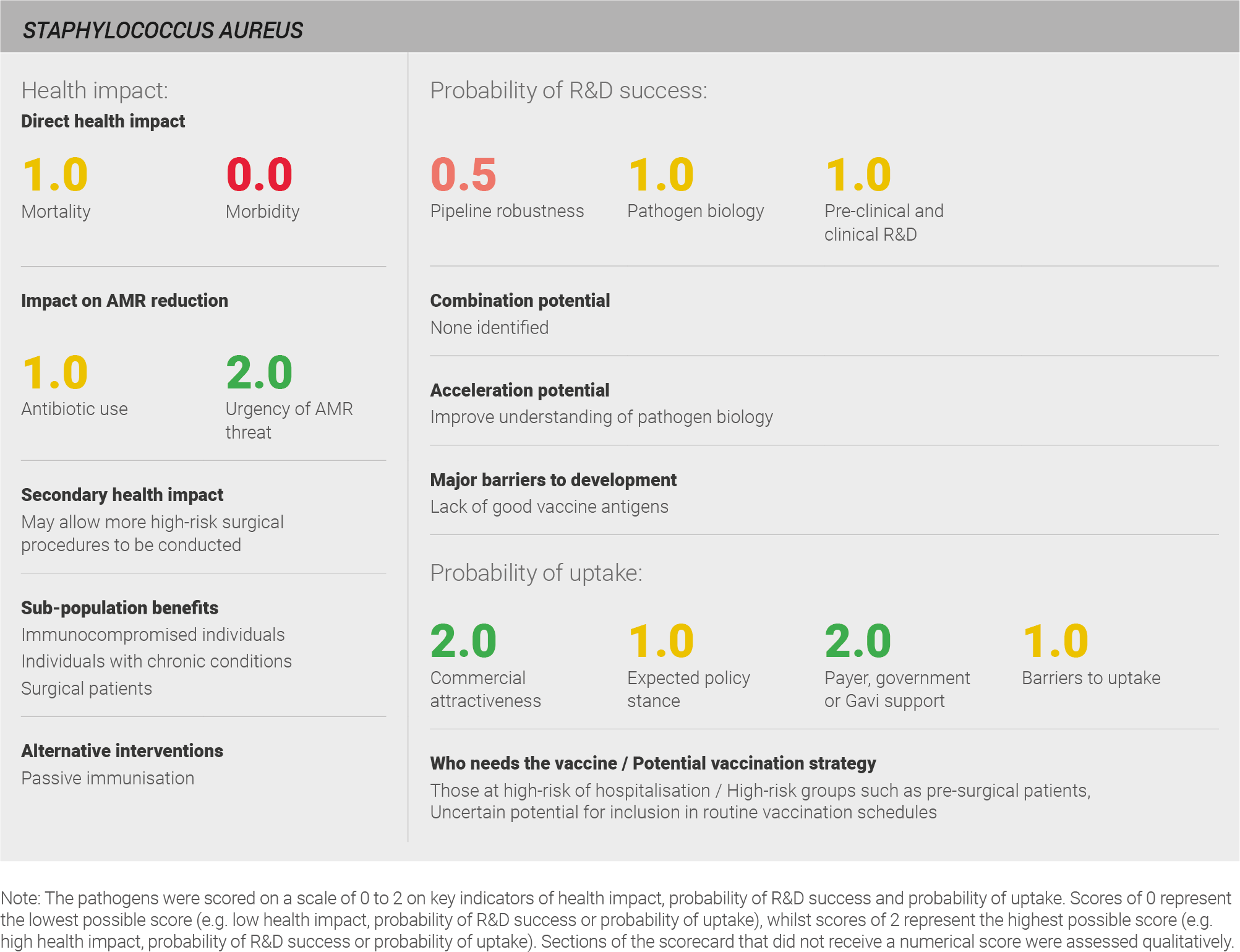
Direct health impact
Global data on disease burden is not available from the IHME, WHO or in the research literature, but data suggests that S. aureus causes significant disease burden. Globally, S. aureus is responsible for approximately 30% of cases of cellulitis 384, 30% of cases of endocarditis 385, 10% of cases of pneumonia 33, and 3% of cases of meningitis 381. Given the lack of direct data on the burden of S. aureus, it is challenging to precisely assess the global burden with confidence. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was categorised as medium (score of 1 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Secondary health impact
The secondary health impact of a S. aureus vaccine would likely be greatest for patients undergoing surgery. An effective vaccine may decrease the risk of post-operative infections, giving physicians greater confidence in recommending surgery where patients are likely to derive benefit. As one expert explains, “it is important to have vaccines for pathogens that are problematic in the hospital like Staph aureus” 28.
Sub-population benefits
The sub-populations most likely to benefit from a vaccine against S. aureus are immunocompromised individuals and those with chronic health conditions, amongst whom the infection is most severe 386. Young children and the elderly may also benefit. Finally, because a significant portion of surgical site infections are caused by S. aureus 2 – resulting in prolonged hospital stays and increased morbidity and mortality – surgical patients would also benefit from a vaccine.
Antibiotic use
First-line antibiotic treatment for S. aureus infection includes penicillins and cephalosporins. The treatment course is typically seven days but varies depending on the specific condition. Treatment of endocarditis, for example, can require a one-month course of antibiotic treatment.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score 1 out of 2). This estimate is based on an annual incidence of ~35 million LRTIs treated with a seven day course of antibiotics, ~18 million cellulitis cases treated with a five day course of antibiotics and ~400,000 endocarditis cases treated with a one month course of antibiotics.
Urgency of AMR threat
The WHO and the CDC have both expressed concern about the future of S. aureus treatment. Both have placed methicillin-resistant S. aureus (MRSA) on their AMR watch lists, and the WHO has listed S. aureus as a ‘high’ priority for development of new antibiotics 6. The CDC has also listed vancomycin resistant S. aureus (VRSA) as a ‘concerning’ threat 7.
MRSA was first reported shortly after the introduction of methicillin in 1961, but it was uncommon outside of a healthcare environment until the 1990s 387. Methicillin resistance is now found in approximately 50% of all staphylococcus infections 380. Vancomycin is currently the main recourse for combating MRSA, but strains of S. aureus with reduced susceptibility to vancomycin have also developed. Vancomycin intermediate S. aureus (VISA) was first described in 1996 388 and has now been documented across most of the globe 388–390. Acquired vancomycin resistance is currently rare, but at least 14 cases of VRSA have been reported in the United States 391. Furthermore, colonisation with MRSA and VRE is very common, and the potential for horizontal transfer of the vanA gene raises the risk of more extensive VRSA development 391. Finally, resistance to daptomycin – a last line treatment for S. aureus – has been reported 392,393.
Scoring: Based on the above analysis, the urgency of AMR threat was categorised as high (score of 2 out of 2).
Pipeline robustness
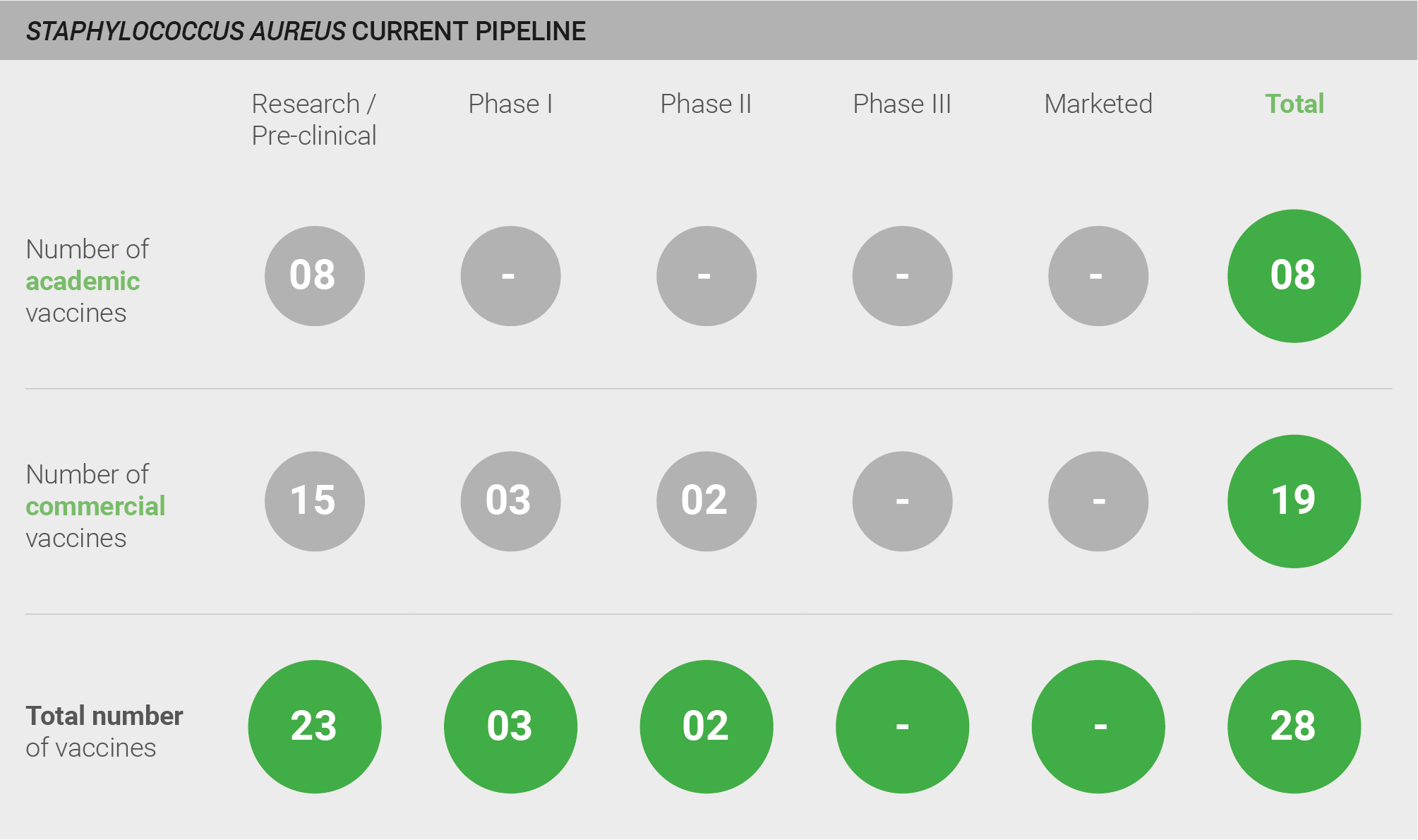
The S. aureus vaccine pipeline was categorised as weak. Although the S. aureus vaccine pipeline is relatively active – with one expert stating that “the Staph market is busy with several companies developing vaccines” – experts are predominately not optimistic about the probability of success for vaccines in the current pipeline. The pipeline comprises a total of 27 vaccine candidates: 23 in pre-clinical development, two in Phase I trials, and two in Phase II trials. However, despite strong commitment from industry, there are no marketed vaccines and experts agree that development will be difficult. The most advanced vaccines currently in development are Pfizer’s four-antigen vaccine (SA4Ag), which is in a Phase II adaptive trial and has been granted FDA Fast Track designation, and NovaDigm’s NDV-3, which is also in Phase II trials. The probability of success for these vaccines is unclear; notably, a Phase III trial of the Merck 710 vaccine was recently halted because of safety concerns (discussed in more detail in a subsequent section) 394. One expert explains that whilst there is “at least 50/50 chance to get a vaccine to market, we’ve seen vaccines failing in late clinical trials and we don’t know the reason why” 28.
Other experts believe that the number of vaccines in the S. aureus pipeline might not accurately reflect the state of knowledge about this pathogen. Having experienced expensive failures of promising vaccines, it is possible that the development of successful candidates now depends on better understanding of the pathogen and its interaction with hosts 395.
Scoring: Based on the above analysis, pipeline robustness was categorised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
S. aureus can exist within the normal human flora and has evolved a number of strategies to colonise and evade host immunity as a result 396. Notably, prior S. aureus infection does not provide protection against re-infection 397, but infections among carriers may be less severe, indicating that prolonged colonisation leads to a limited form of immunity 398. Adults typically have pre-existing S. aureus-specific antibodies, including antibodies against capsule and clumping factor A, but these typically do not have opsonophagocytic or neutralising properties and do not provide protection against infection 386.
To date, candidates that have seemed promising in animal models have not yet demonstrated efficacy in human trials 386. However, several vaccine targets have been identified. Vaccine candidates have targeted individual cell surface components, such as the S. aureus capsule and extracellular polysaccharides, and cell wall associated proteins including attachment proteins, invasion proteins, and receptors. Given the failure of single antigen approaches, vaccine development currently focuses on multi-antigen approaches. For example, Pfizer’s SA4Ag candidate includes clumping factor A (ClfA), the manganese transport component (MntC), and capsular polysaccharides 5 and 8 conjugated to CRM197 386.
Even with vaccine candidates identified, critical gaps in the understanding of pathogen biology persist. Mechanisms for phagocyte-mediated killing of S. aureus remain to be established and are likely essential for the development of a successful vaccine, as polysaccharides do not seem to be essential for colonisation or invasive disease. One expert emphasises that a clearer understanding of pathogen biology is needed to facilitate vaccine development, stating, “[the] biology of Staph aureus is incompletely understood […] in some areas you get more virulence […] and we don’t understand why” 28.
Scoring: Based on the above analysis, pathogen biology was categorised as medium (score of 1 out of 2).
Pre-clinical and clinical R&D
Animal models exist for S. aureus infection and have provided some useful insights but also have important limitations. Whilst mouse models have proven extremely useful in determining the role of many virulence factors and identifying host pathways that contribute to infection, they do not appear to predict the success of vaccines in humans 387. One reason for this could be that S. aureus produces a number of virulence factors that have high species specificity toward the human molecular counterpart they target 399. The next generation of animal models may be more successful; humanised mice have been developed that have increased susceptibility to S. aureus. However, even with improved animal models, some aspects of pathogen-host interactions require further investigation; notably, protective immunity against S. aureus is not completely understood 387.
Clinical development programmes for S. aureus will involve some key challenges. The initial target population for vaccination is patients presenting for elective surgeries. However, targeting only pre-surgical patients may not reduce infection rates as much as expected. These patients may have already been exposed to antibiotics, as well as chlorohexidine/murpirocin (fusidic acid) treatment in an attempt to reduce the risk of infection. Reducing this risk further may be difficult. Experts ask: “are we trying to reduce the irreducible?” 28. Other high-risk groups comprise patients at high risk of infection, including immunocompromised patients and those with chronic conditions. Less healthy populations might have difficulties mounting an effective immune response after vaccination, and it is not clear that the findings from immunocompromised patients can be generalised to other high-risk groups. Experts explain, “with frail patients […] we may need something that is more potent than with other pathogens at the community level” 28.
The lack of established correlates of protection also poses some challenges to trial design; understanding what immune responses predict protection would help simplify outcome measures included in clinical trials.
In summary, additional investigations are needed to help guide clinical trial design. Prospective studies are needed across a number of different surgery procedures and comorbidities to refine a target population for clinical development of a S. aureus vaccine, and further studies of infection rates are needed to understand optimal trial design and identify the numbers needed to adequately power a trial to detect an effect 58.
Trials conducted in humans to date have yielded mixed results and provide reasons for both caution and optimism regarding the probability of developing an effective S. aureus vaccine. However, whilst experts express concerns including “I am very worried about the S. aureus vaccine,” 28, both recent and planned future trials provide grounds for optimism, as one expert emphasises “I wouldn’t give up [on a S. aureus vaccine]”.
The recent failure of Merck’s Phase III trial of its V710 vaccine highlights the difficulties in developing vaccines for S. aureus. This trial was a double-blind, randomised, placebo-controlled trial among 8031 surgical patients aged 18 years or older who were scheduled for surgery involving full median sternotomy at 165 sites in 26 countries. The trial objective was to determine whether V710, administered 14-60 days prior to surgery, reduced postoperative S. aureus infection. The trial was halted after the second interim analysis because mortality rates in patients with staphylococcal infections were significantly higher in the intervention arm, though the difference in overall mortality between the trial arms was not statistically significant 394. A subsequent analysis of the study results identified three coincident factors that predisposed patients to mortality: low pre-vaccination IL-2 levels, receipt of the V710 vaccine, and infection with S. aureus 400. The identification of host factors that may adversely affect the safety of an S. aureus vaccine has contributed to experts’ concerns that development of a safe and effective vaccine could prove challenging.
Subsequent trials of other vaccine candidates have provided reasons for optimism despite the need to discontinue the trial of V710. The four-antigen S. aureus candidate SA4Ag was examined in a Phase II trial initiated in 2015. This was a double-blind, placebo-controlled randomised trial to evaluate safety, dosing, and immunogenicity of the SA4Ag vaccine. The trial enrolled 454 healthy adults aged 18-85 years scheduled to undergo elective open spinal fusion surgery. Single dose vaccination safely induced an immune response that was durable through a 12-month follow-up period 401.
NovaDigm also has a candidate vaccine in clinical trials; the company announced its Phase IIa trial of NDV-3 in April 2018. This is a double-blind, placebo-controlled, randomised trial to evaluate safety, immunogenicity, and efficacy of NDV-3 in reducing nasal and oral acquisition of S. aureus. NovaDigm plans to recruit approximately 400 United States Army Infantry trainees at Fort Benning, Georgia 402 with follow-up to occur throughout the 14-week training cycle to assess S. aureus colonisation status.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
The probable initial target population for an S. aureus vaccine will comprise those presenting for elective surgeries, with eventual expansion to other high-risk groups and routine vaccination in the elderly. Other high-risk groups comprise immune-compromised patients, individuals with chronic conditions, patients undergoing non-elective surgeries, and the very young. However, some experts suggest that a routine vaccination strategy “is more suitable [than a targeted approach]” 6. Indeed, one expert notes that “the consequences of these intensive surgical procedures and the burden globally is so high that one, in the longer run, could accept universal immunisation” 28.
The WHO and CDC concur that the spread antimicrobial resistant strains of S. aureus is concerning. In 2016, the World Economic Forum published an article on vaccines for S. aureus, highlighting acceptance that vaccination may contribute to containing the spread of the pathogen 28,403. In general, experts suggest that there is a likelihood of policy support; with one stating that “the WHO would have to support a vaccine because their member states will have Staphylococcus aureus problems” 6. However, following the meeting of the WHO’s Product Development for Vaccines Advisory committee (PDVAC) meeting in 2017, no specific advocacy for the development of a S. aureus vaccine has arisen. Further, some experts suggest that barriers to identifying patients who may present for surgeries in a timely manner present a problem that would need to be overcome in order to garner support. One expert explains “the issue is, how do you vaccinate before people enter into the high-risk population?” 6.
Scoring: Based on the above analysis, the expected policy stance was characterised as medium (score of 1 out of 2).
Likelihood of payer, government, or Gavi support
Payers in high-income countries are likely to be willing to pay for a vaccine targeting S. aureus because of the costs associated with hospital-acquired S. aureus infections. Extensive media devoted to the pathogen and the high economic burden it presents may also drive a favourable cost-effectiveness assessment. Regulators also appear supportive and have agreed to adaptive trials to accelerate development in elective surgery patients 6. However, regulatory barriers may exist regarding the burden of evidence needed to add new populations eligible for the vaccine, particularly if those populations are immunocompromised. Similarly, media attention and the likelihood of a favourable cost-effectiveness assessment suggest there may be support for vaccination against S. aureus in middle-income countries.
Support for a vaccine targeting S. aureus is unlikely in low-income countries. The evidence of disease burden in Gavi-eligible countries is likely insufficient for Gavi to make a positive investment decision. Therefore, Gavi support is unlikely, and novel financing mechanisms would be required.
Scoring: Based on the above analysis, likelihood of payer, government, or Gavi support was characterised as high (score of 2 out of 2).
Barriers to uptake
Some logistical factors will present some challenges to implementation of a vaccination programme. The initial target population – surgical patients – is in touch with healthcare services, and this is likely to drive adoption. However, a vaccination touchpoint would still need to be incorporated into the existing bundle of pre-surgical preparations, including the assessment of fitness for surgery. A new programme would need to be built for every high-risk population identified, as additional populations may have less contact with healthcare services or require a different type of touchpoint.
Scoring: Based on the above analysis, barriers to uptake were characterised as medium (score 1 out of 2).
Commercial attractiveness
Development of an S. aureus vaccine may be an attractive commercial opportunity given the burden of disease in high-income countries and increasing concerns about AMR. These factors provide what one expert described as “enormous motivation from pharma to reduce post-surgical severe Staph disease”28.
Scoring: Based on the above analysis, commercial attractiveness was characterised as high (score 2 out of 2).
Primary recommendation
Investment in pre-clinical research will be critical for development of a vaccine against S. aureus. Specific areas that warrant investigation are the identification of conserved non-capsular antigens that could be part of a multivalent vaccine, and the development of novel technologies such as mucosal adjuvants that are likely to facilitate vaccine development. Given the commensal nature of the pathogen, pre-clinical research should also seek to better understand the potential effect of vaccines on nasal flora.
Secondary recommendations
Alternative treatments for S. aureus infection merit exploration. In particular, the induction of passive immunity using monoclonal antibodies or other approaches may help to overcome the challenges involved in developing vaccines for hospital-acquired infections. These include the immunocompromised nature of many hospital patients, and the resultant reduced probability of mounting an effective immune response to a vaccine. Further, as many hospital admissions are unplanned, and vaccines require significant lead times to allow for the development of immunity, passive immunity may be preferred. However, development of monoclonal antibodies is costly and many of the development challenges are the same as for vaccines, including cost and the need to identify appropriate antigens.
A clearer understanding of the epidemiology and disease burden at a global and regional level with a specific focus on the burden of S. aureus infection in low-income countries and middle-income countries would help inform policy-making. Currently, no WHO or IHME estimates exist for S. aureus and little is known about the incidence and burden of S. aureus in low-income countries and newly industrialised regions. Experts believe that in these regions S. aureus has a similar impact to that seen in high-income countries 28, but existing studies in these regions yield diverse estimates that are likely to be confounded by incomplete case ascertainment. Greater clarity is therefore needed to characterise the potential cost-effectiveness of an S. aureus vaccine.
Given that current animal models do not appear to predict the success of vaccines in humans, it is also recommend that development of next-generation humanised animal models that promise to better mirror human disease and improve candidate translatability in early clinical trials is prioritised.