Shigella spp
Shigella is one of the most common bacterial causes of diarrhoeal illnesses. It is responsible for ~ 200,000 deaths per year, primarily in low and middle-income countries. Enteric diseases have a massive impact on AMR due to the volume of antimicrobials used to treat them. Experts believe a Shigella vaccine is feasible due to defined and promising target antigens and feasible technical R&D. A Shigella vaccine would have high probability of uptake across low and middle-income countries, primarily due to likelihood of Gavi support, where incidence is highest.
Shigella falls into a cluster of pathogens for which bringing a vaccine to market is the priority. The primary recommendation is to accelerate clinical development. The secondary recommendation is to incentivise the development of multi-pathogen/combination vaccines.
Shigella are Gram-negative, non-motile bacteria closely related to Escherichia coli (E. coli). There are four different species of Shigella (S. sonnei, S. flexneri, S. boydii, and S. dysenteriae 365) that can cause diarrhoeal disease. Shigella is transmitted via the faeco-oral route, through direct person-to-person or sexual contact, or indirectly through contaminated food, water, or fomites 366.
Typical symptoms of Shigella infection include diarrhoeal disease (frequent, loose stools with blood and mucus), fever, and abdominal cramps, and pain 366. Groups at highest risk for Shigella include young children, travellers, men who have sex with men, and people whose immune systems are weakened due to illness or medical treatment 367.
Shigella is more common in low-income countries than in middle- or high-income countries. Mortality from Shigella is highest in the WHO African region (10 per 100,000) and South East Asia (4 per 100,000) 31. Over 190 million cases of Shigella occur globally per year 368.
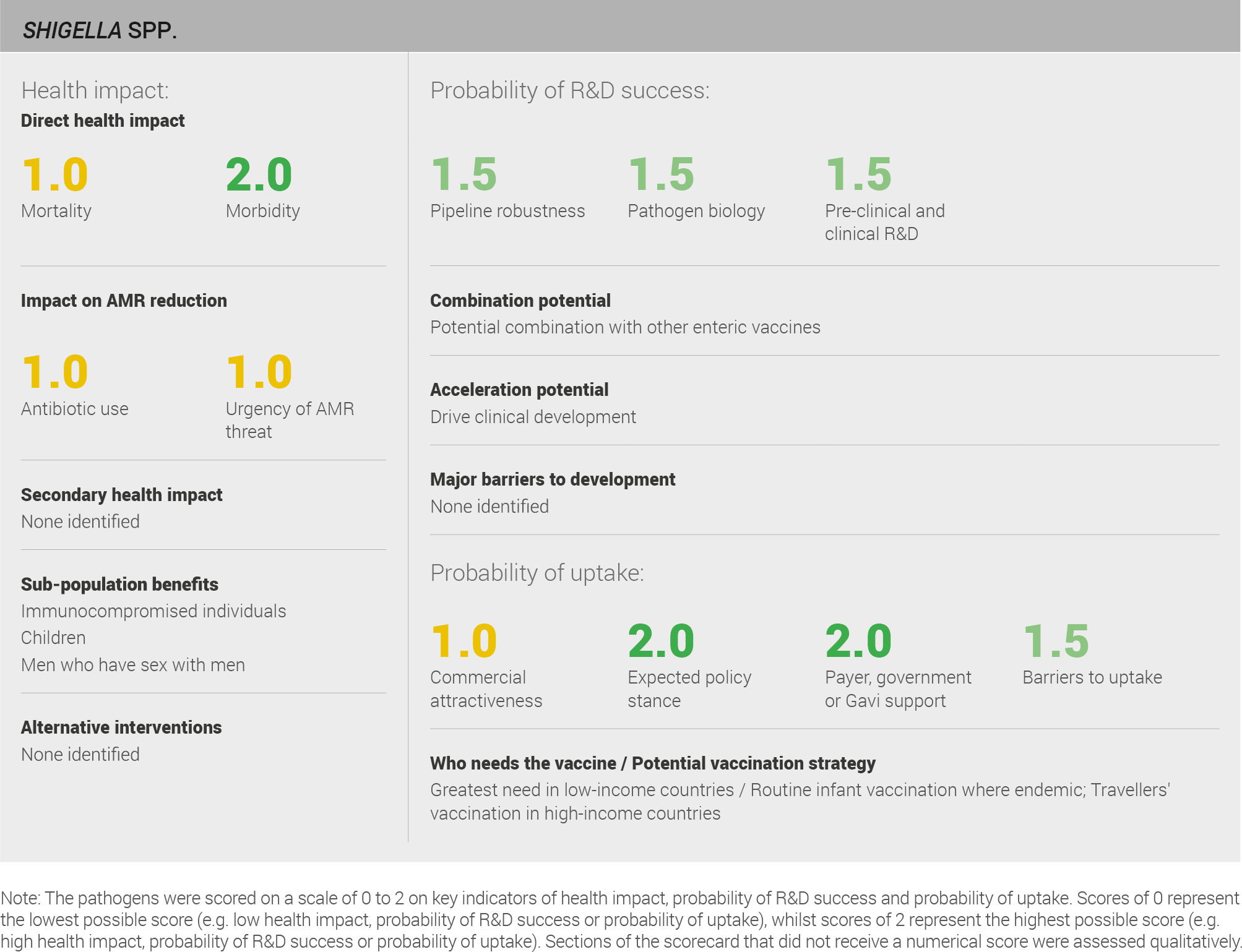
Direct health impact
Data on mortality and morbidity were taken from the IHME 2016 estimates. Shigella infection is associated with an estimated 212,000 deaths and 450,000 years lived with disability annually 31. This source uses a defined methodology and is used in the global health community. The data can therefore be viewed with a reasonable level of confidence. Additional support comes from the GEMS study that found in sub-Saharan Africa and South Asia, Shigella infection was among the top four causes of potentially life-threatening diarrheal illness among children less than five years old brought to a center for treatment of diarrhea 363,369.
Scoring: Based on the above analysis, mortality was categorised as medium (score of 1 out of 2). Morbidity was categorised as high (score of 2 out of 2).
Secondary health impact
The secondary health impact of a Shigella vaccine is unclear; there is some debate regarding impact of diarrhoeal disease on growth trajectories for children, especially those with multiple diarrhoeal episodes 67,68. However, it is possible that these children experience catch-up growth and return to normal growth trajectories 69.
Sub-population benefits
The groups who will benefit the most from a Shigella vaccine are those at greatest risk of infection: immunocompromised patients, including those with HIV and cancer patients; men who have sex with men; and young children.
Antibiotic use
First line treatment varies depending on regional resistance patterns but is typically a course of treatment with fluoroquinolone. Although treatment duration varies; a three-day course is typical 370.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score 1 out of 2). This estimate is based on an annual incidence of ~50 million Shigellosis cases treated with a three day course of antibiotics.
Urgency of AMR threat
Shigella is listed as ‘medium’ in the WHO priority list of R&D for new antibiotics and as a ‘serious’ threat in the CDC’s list of biggest threats from AMR. In Asia and Africa, 65-85% of Shigella infections are resistant to nalidixic acid and trimethoprim-sulfamethoxazole, and 20-30% are resistant to fluoroquinolones 370. A strain in Vietnam has displayed resistance to third generation cephalosporins and fluoroquinolones 370, and strains in Bangladesh requiring treatment with the last-line antibiotic meropenem have been reported 371.
Scoring: Based on the above analysis, AMR threat was categorised as medium (score of 1 out of 2).
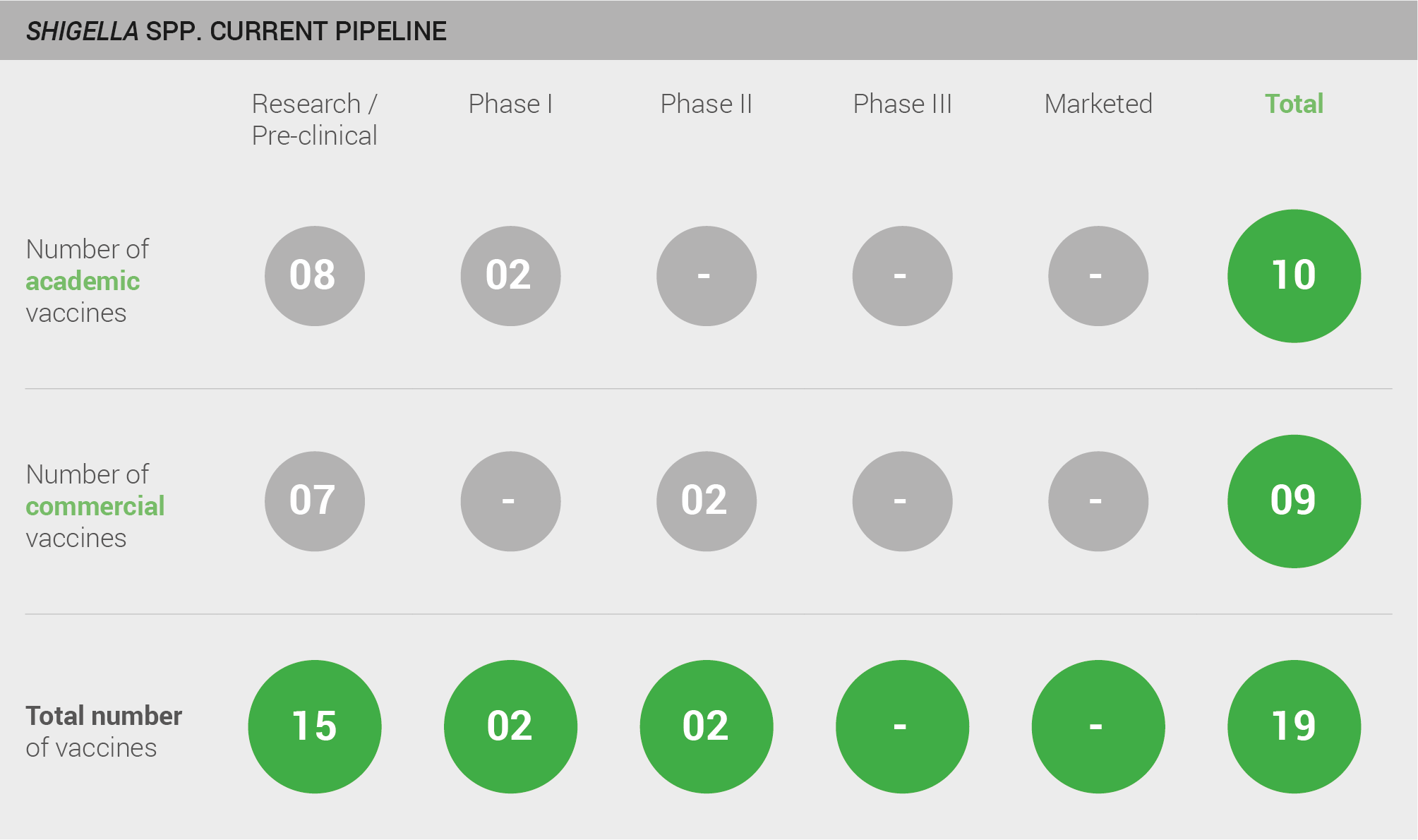
Pipeliness robustness
The pipeline for a Shigella vaccine includes a moderate number of candidates. A total of 19 vaccines are in development; 15 are in pre-clinical development, two are in Phase I, and two are in Phase II.
Experts strongly believe that a vaccine for Shigella will be successfully developed and marketed; however, given the length of time that development takes, it will likely be five to ten years before a vaccine is licensed. One expert notes “GSK has two different candidates in Phase II; however, a marketed vaccine is not expected earlier than in seven to 10 years, given that these vaccines candidates are still monovalent and will likely be optimised before progressing to late stage clinical trials” 28.
Scoring: Based on the above analysis, pipeline robustness was categorised as fairly high (score of 1.5 out of 2).
Current pipeline
Pathogen biology
Serotype-specific natural immunity is induced by infection. However, the large variety of Shigella species and serotypes (four major species and 50 different serotypes) make reinfections possible. Serum and mucosal antibody responses to Shigella are predominantly homologous responses directed against a serotype-specific Shigella LPS-associated O antigen 371. Immune responses to Shigella are robust and lead to the induction of memory-B cell responses. However, evidence of their ability to cross-protect against diverse serotypes is inconclusive 371. Furthermore, systemic and mucosal responses against conserved invasion plasmid antigens (Ipa B, Ipa D) do not seem to be very immunogenic in the natural setting 371. It is therefore likely that multivalent vaccines will be needed to prevent shigellosis.
Because natural Shigella immunity is serotype-specific, LPS-associated O-specific polysaccharide (O-SP) antigens are logical possible vaccine targets. Targeting the O-SP antigens of S. flexneri 2a, 3a, and 6 as well as S. sonnei should cover the majority of all Shigella illnesses and protect against 64% of Shigella strains directly and 88% of all strains when considering cross-protection. One expert confirmed the value of this approach by explaining “The perfect strain selection depends a bit on your geographic location, but by covering four serotypes you could cover 75% of the cases” 28.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly high (score of 1.5 out of 2).
Pre-clinical and clinical R&D
As humans are the only natural host for Shigella, it has been difficult to establish predictive animal models. Several animal challenge models currently exist, but “none mirrors human infections well” 28 according to experts. Current models include a guinea pig keratoconjunctivitis model, a murine pulmonary model, a cynomolgus monkey S. dysenteriae 1 model, and a guinea pig and piglet oral and intrarectal challenge model. Immunoassays that can correlate clinical severity with immunological status also exist. Two types of assays are in development, one is an opsonophagocytic assay and the other is a serum bactericidal assay. Initial data from these models show feasibility for Shigella vaccine development.
Human controlled infection models have been established using either the S. sonnei strain 53G or the S. flexneri 2a strain 2457T. Given the lack of appropriate animal models, these have proven to be very useful for early assessment of vaccine efficacy 372–375. However, experts caution that “the flipside is that challenge models slow vaccine development because people want to [use challenge models to] test in North American adults and not in kids who are affected” 28.
Field efficacy trials should be possible as incidence of Shigella infection is high in endemic regions, allowing for adequate trial enrollment. Also, a new quantitative PCR assay is available that is more sensitive than traditional culture methods. This will provide critical support for R&D efforts as traditional culture methods may have seriously underestimated the burden of Shigella-associated illness 363.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as high (score of 2 out of 2).
Expected policy stance
A Shigella vaccine would likely be offered as a routine childhood vaccination in endemic regions, predominately in low- and middle-income countries, and as an elective travellers’ vaccine. Policymakers are likely to support a Shigella vaccine, primarily to reduce the high mortality burden, and WHO is currently preparing a Shigella vaccine pathways document 376. An expert characterises the choice to support a Shigella vaccine as “straightforward because this is a high burden of disease that affects children” 28. Experts acknowledge that enteric diseases have a massive impact on the development of AMR due to the high quantities of antimicrobials used in their treatment. Therefore, even a “partially efficacious vaccine that reduces the severity of the disease rather than preventing the disease could have a huge impact as patients would seek antimicrobial treatment less often”.
Scoring: Based on the above analysis, the expected policy stance was categorised as high (score of 2 out of 2).
Likelihood of payer, government, or Gavi support
High-income countries would likely treat a Shigella vaccine similarly to the typhoid fever vaccine, which is recommended as a travellers’ vaccine 360,377. In middle-income countries the incidence and mortality of Shigella infection are high and governments and payers are likely to endorse a vaccine against Shigella. The probability of investment by Gavi is high because of the high mortality of Shigella in low-income countries.
Scoring: Based on the above analysis, likelihood of payer, government, or Gavi support was categorised as high (score of 2 out of 2).
Barriers to uptake
Relatively few barriers will limit uptake of a Shigella vaccine. A vaccine would not require a new vaccination touchpoint, as it would likely be delivered as part of childhood vaccination programmes or travellers’ vaccine appointment schedules. However, since the greatest burden of disease is in low-income countries, additional costs may be incurred for development of supply chain and storage. New clinical practices would not be required as childhood vaccines and travellers’ vaccines are routinely recommended by clinicians.
Scoring: Based on the above analysis, barriers to uptake were categorised as fairly low (score of 1.5 out of 2).
Commercial attractiveness
Although this is not an inherently attractive commercial market, there is robust global health interest in a Shigella vaccine. Therefore, R&D is being subsidised by funders like the Gates Foundation and procurement will likely be supported by Gavi.
Scoring: Based on the above analysis, commercial attractiveness was categorised as medium (score of 1 out of 2).
Primary recommendation
The primary recommendation for Shigella is to accelerate clinical development. An expert states, “we should get the vaccine into kids [the target population] as quickly as possible, as only this data can really support a go or no-go decision” 28. Key funders of enteric disease research should be encouraged to support large Phase III trials for promising clinical vaccine candidates. Given the lack of a strong commercial market, additional support via market shaping mechanisms should be considered to accelerate clinical development and help bring vaccines to market. Opportunities for funders to strategically coordinate efforts would allow them to pool resources and fund later-stage trials for Shigella.
Secondary recommendations
Development of combination vaccines should be incentivised. ETEC vaccines were mentioned by experts as possible combination candidates, and in the longer term a combination vaccine could include other enteric diseases 28. Funders should support R&D to explore combination potential given the strong policy interest in enteric vaccine combinations.