Pseudomonas aeruginosa
Pseudomonas aeruginosa (P. aeruginosa) is predominantly hospital-acquired and patients with compromised immune systems are predisposed to infection. Patients with chronic lung diseases such as cystic fibrosis and bronchiectasis are most at risk of colonisation. Although morbidity and mortality are thought to be low, AMR is an immediate concern as P. aeruginosa is inherently drug-resistant and pan-resistant strains have been reported. Previous attempts to develop vaccines have been unsuccessful, and pathogen biology is not well understood. Payer support for high-risk groups in high-income countries is likely but disease burden in low and middle-income countries is not well-defined.
P. aeruginosa falls into a cluster of pathogens for which advancing early R&D is the priority. The primary recommendation is to support pre-clinical research. The secondary recommendation is to explore alternative treatments and prevention strategies and to better understand the burden, epidemiology and transmission of the pathogen.
P. aeruginosa is a Gram-negative bacterium that primarily causes hospital-acquired infections. Colonisation with P. aeruginosa, however, can be community-acquired or hospital-acquired.
Immunocompromised patients infected with P. aeruginosa are at risk of several clinical syndromes including pneumonia, post-burns skin infection, and post-surgical infections, such as surgical site infections and urinary tract infections 296. Community-acquired infections associated with P. aeruginosa include folliculitis and pneumonia.
P. aeruginosa is transmitted through touch and contaminated equipment 297. In the case of lung colonisation, the pathogen can also be transmitted through air droplets spread by patients who are already infected, or through animal reservoirs 298. The presentation of P. aeruginosa is dependent on clinical syndrome. Clinical features of pneumonia include pyrexia, headache, malaise, and dry cough 296. Surgical site infections can show purulent drainage, pain, swelling, erythema, heat, wound dehiscence, fever, and abscess 296.
P. aeruginosa is known to be present worldwide 299. However, there is insufficient epidemiological information to determine the burden of disease. In some sub-populations, it is possible to determine the geographic distribution of clinical syndromes that are associated with increased incidence of P. aeruginosa infection. For example, the prevalence of cystic fibrosis is highest in Europe, North America and Australia 300.
Direct health impact
Robust global data on disease burden is not available. Globally, P. aeruginosa is estimated to be responsible for approximately 3% of pneumonia cases 33, 2% of urinary tract infection cases 82, and less than 1% of neonatal meningitis cases 34. The level of confidence in these estimates is relatively low because P. aeruginosa infections are not reported by the WHO or IHME and no publications that report the global burden of this pathogen were found. A full methodology for this assessment can be found in the appendix.
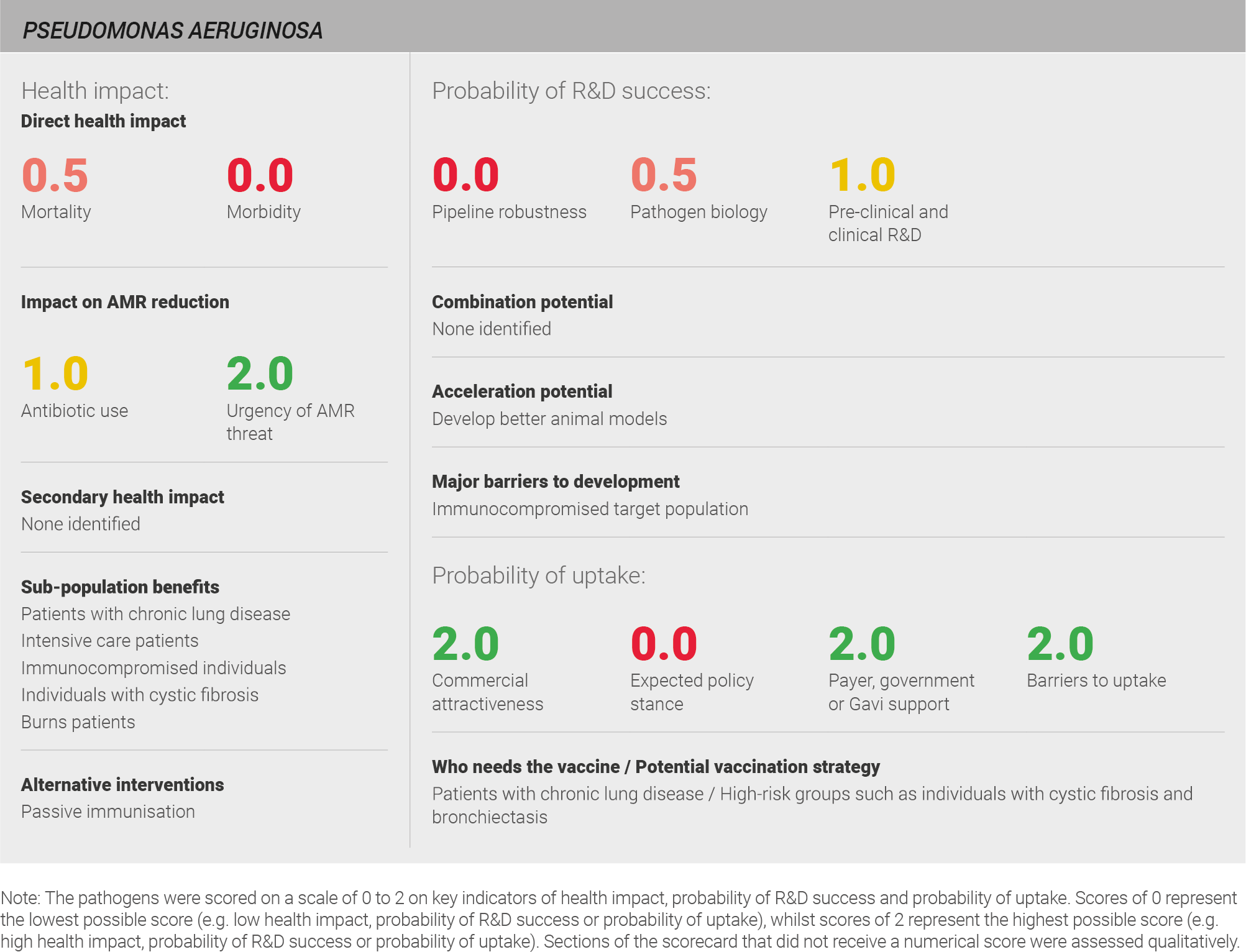
Scoring: Based on the above analysis, mortality was characterised as low (score of 0 out of 2) and morbidity was categorised as fairly low (score of 0.5 out of 2).
Sub-population benefits
Patients with chronic lung disease are at risk of P. aeruginosa colonisation 301,302,303 and would benefit from a vaccine.
Antibiotic use
Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles. Regimens vary in length but often involve a two-week course of a broad spectrum antibiotic for pneumonia and a five-day course of a fluoroquinolone antibiotic for urinary tract infection 304,305. However, P. aeruginosa infection is not easy to treat. One expert notes “P. aeruginosa is difficult to reach with antibiotics even if appropriate activation of the immune system [is achieved]” 28. This is because part of the process of P. aeruginosa colonisation involves the pathogen reducing the expression of virulence factors and forming biofilms 306,307. These actions diminish the ability of antibiotics to have bacteriostatic or bactericidal effects.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~ nine million LRTIs treated with a two week course of antibiotics, and ~five million UTIs treated with a five day course of antibiotics.
Urgency of AMR threat
Both the WHO and CDC have expressed strong concern about antibiotic treatments for P. aeruginosa. The WHO lists P. aeruginosa as ‘critical’ in its priority list of R&D for new antibiotics 31 and the CDC lists it as a ‘serious’ threat in its list of biggest threats from AMR 7. P. aeruginosa is inherently drug-resistant for two reasons: first, it has constitutive expression of certain proteins which enable resistance to antibiotics, for example, expression of AmpC beta-lactamase and efflux pumps for penicillin resistance, and second, the outer membrane of P. aeruginosa has low permeability to antibiotics 308. Additionally, P. aeruginosa can develop additional resistance during treatment, due to the ability to easily acquire many escape mechanisms 309,310. Some strains are resistant to more than three classes of antibiotic and pan-drug resistant strains have been reported 310.
Scoring: Based on the above analysis, the urgency of AMR threat was categorised as high (score of 2 out of 2).
Pipeline robustness
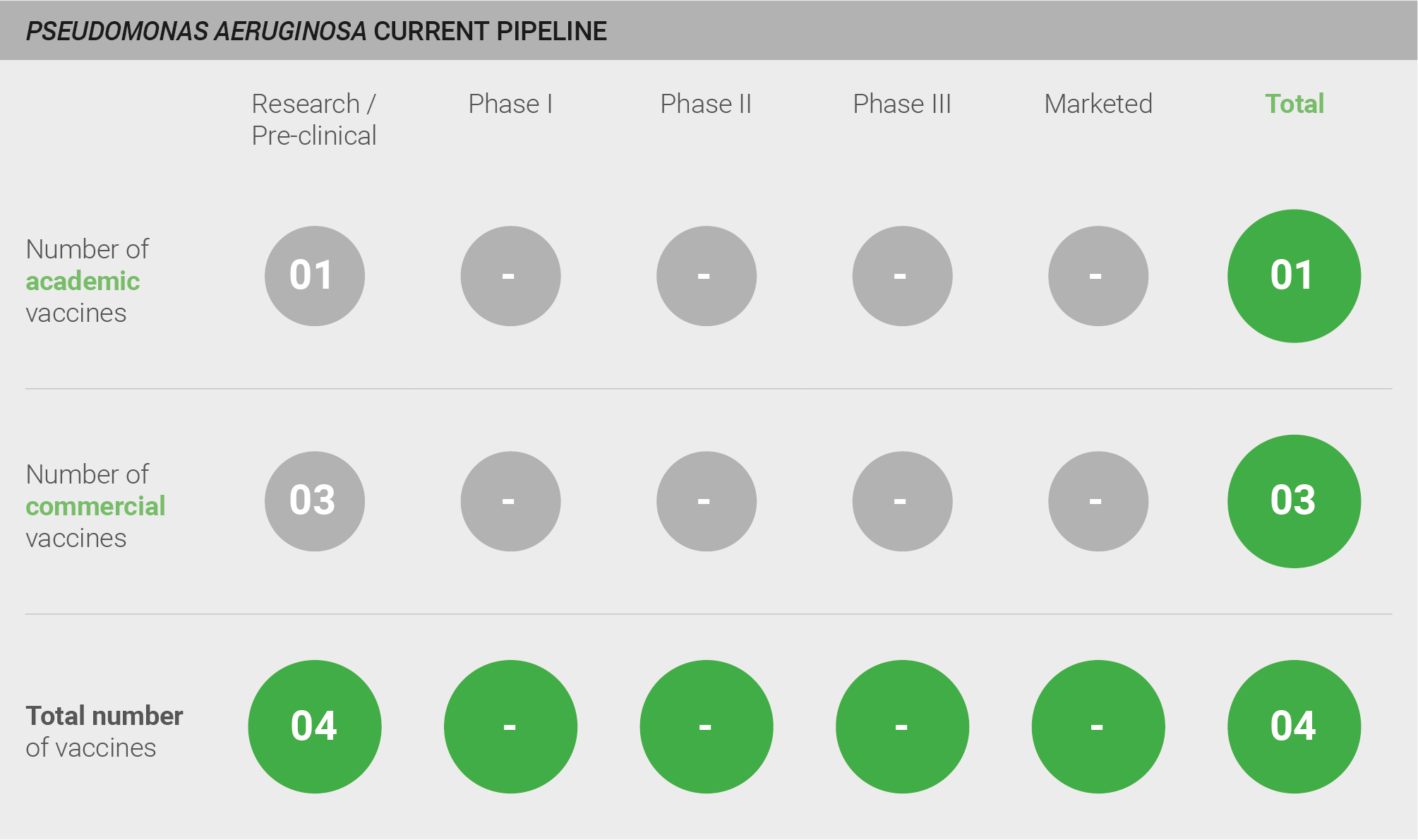
The pipeline for vaccines against P. aeruginosa is weak, with only four vaccines currently in pre-clinical development. Other historic clinical-stage candidates are no longer undergoing active development.
Scoring: Based on the above analysis, the pipeline was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
Current research does not provide a clear understanding of natural immunity to P. aeruginosa. Infection with P. aeruginosa induces an innate immune response in healthy individuals 311. However, the adaptive immune response in cases of chronic infection can cause airway remodelling, which is maladaptive and does not result in pathogen clearance 312.
One way to infer the extent of natural immunity is to examine cross-infection of different strains of P. aeruginosa in cystic fibrosis patients. Cross-infection is known to occur with hyper-transmissible strains and between siblings with cystic fibrosis 313. It is difficult to infer the extent of natural immunity, since once one strain of P. aeruginosa colonises a patient, it adapts to the host environment, for example through becoming less virulent, and establishing biofilms 314,315. This prevents further strains from establishing themselves 316. Furthermore, it is difficult to interpret data regarding reinfection from patients with chronic lung diseases, as these patients often do not readily clear a first infection and because they have a maladaptive response to colonisation which contributes to difficulty clearing pathogens. For example in individuals with cystic fibrosis, there is impaired bacterial ingestion and bacteria are able to bind more easily to viscous mucus 317. Consequently, it is difficult to know if the subsequent infection is a new infection or a prior infection that was not completely cleared.
Vaccine targets are extensively characterised for P. aeruginosa including LPS O-antigens, outer membrane and secreted protein targets318. However, since P. aeruginosa has many inherent escape mechanisms, an effective vaccine will have to encompass many targets. 318. A selection of these mechanisms include targeting opsonic antibodies, anti-toxin antibodies, anti-virulence antibodies and potentially T cell immunomodulation 318. The need to target multiple mechanisms makes vaccine development more challenging.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
A variety of different animal models are in place for hospital-acquired pneumonia. These include “one-hit” (single insult) acute pneumonia models in rats and mice, ventilator-associated pneumonia models in piglets, rats, and mice, and a model using agar beads to mimic the biofilm matrix of cystic fibrosis 319. However, these have often failed to predict efficacy in humans. Animal models are inconsistent and higher infecting doses are rapidly lethal to the animals they infect, whilst lower infecting doses often resolve rapidly 255.
Other models are invasive and ethically or technically challenging to develop (for example, burn-wound infection model) 255. The development of humanised mice that could serve as an improved animal model is ongoing and will likely address some of the limitations of existing models 319. Despite the limitations of animal models, experts believe enough is known about the immunology of P. aeruginosa to develop a vaccine.
Clinical development of a P. aeruginosa vaccine faces some challenges. No easily accessible correlates of protection have been employed in humans 25. There are no known serum markers of seroconversion which can be used in research or clinical practice and there do not appear to be any human controlled infection models ready to be used in clinical testing. However, trial infrastructure is in place and the target population is well-defined. The most recent Phase II/III trial of a P. aeruginosa vaccine was conducted in 800 mechanically ventilated intensive care unit patients 320,321. This trial was unsuccessful in terms of clinical outcomes, as there was no significant difference in P. aeruginosa infection rates between trial arms.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
Hospitalised patients, patients with compromised immune systems, and patients with lung disease, including those with cystic fibrosis, would benefit from vaccination. A vaccination strategy would likely be based on diagnosis of conditions such as cystic fibrosis or other factors placing patients at risk of infection.
There is a paucity of policy documentation in favour of vaccination against P. aeruginosa, and no current momentum for vaccination in the international policy community. At a meeting on vaccination in older adults convened by the WHO in 2018, P. aeruginosa was mentioned as a pathogen for which AMR may be a reason to consider vaccination 322, but aside from this mention, P. aeruginosa has attracted little attention. Expert opinion suggests that strategies other than targeting patients with long term colonisation would be problematic given the small size of the target population and the low incidence of post-surgical complications 28.
Scoring: Based on the above analysis, expected policy stance was categorised as low (score of 0 out of 2).
Payer, government, or Gavi support
Payers in high-income countries are likely to support vaccination against P. aeruginosa. The high risk of colonisation in lung disease means that that these patients would provide a suitable target population. High P. aeruginosa colonisation rates in cystic fibrosis patients cause high morbidity 323. There are ~70,000 cystic fibrosis patients worldwide 324 predominantly in high-income countries. This target population would likely support a high price point as high prices are tolerated for cystic fibrosis interventions, with some treatments costing over £100,000/year 325. There are also 1-2 million bronchiectasis patients worldwide 326, and a quarter are colonised with P. aeruginosa 301, causing substantial burden in high-income countries 327. Bronchiectasis is a potentially life-shortening, chronic disease with high morbidity. P. aeruginosa infection worsens mortality, morbidity and will result in increased hospitalisations in these patients, hence there is likely to be payer support for this population.
A P. aeruginosa vaccine would be less likely to receive support in middle-income countries given the higher thresholds needed for cost-effectiveness in order to access healthcare funding. Gavi is unlikely to support a vaccine for P. aeruginosa because of low mortality from P. aeruginosa infection.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as high (score of 2 out of 2).
Barriers to uptake
Patients with P. aeruginosa colonisation come from sub-populations with significant disease burden and high levels of engagement and advocacy such as cystic fibrosis patients. These patient groups are likely to overcome any barriers to treatment, as they are highly motivated to take actions to modify disease course.
Although a new healthcare touchpoint would need to be created for vaccination programmes in each target population, vaccination programmes would likely be able to leverage touchpoints at diagnosis of conditions that increase the risk of P. aeruginosa colonisation. Vaccines could therefore be embedded into existing clinical pathways relatively easily.
New vaccination programmes would require engagement with specialist societies and guideline setting bodies to ensure awareness of new programmes and dissemination of guidelines.
Scoring: Based on the above analysis, barriers to uptake was categorised as low (score of 2 out of 2)
Commercial attractiveness
A vaccine targeting P. aeruginosa would likely be commercially attractive because of well-defined target populations, especially patients with chronic lung disease. While mortality and morbidity at the global level is likely low, within target populations it is high and in high-income countries there is likely to be high willingness to pay for treatment.
Scoring: Based on the above analysis, commercial attractiveness was categorised as high (score of 2 out of 2).
Primary recommendation
The primary recommendation is to support pre-clinical research. Existing animal models have limited predictive value for clinical research and can be technically challenging for researchers. The development of humanised animal models may lead to more informative animal studies 255,319. An alternative approach is to test P. aeruginosa vaccines in disease-specific models. For example, in cystic fibrosis, non-murine, non-rodent animal models, such as ferret and pig models, show promise in better replicating human disease 323,328. In bronchiectasis, knowledge of disease aetiology and pathophysiology is incomplete, making development of an animal model challenging 329,330. Given the commensal nature of the pathogen, pre-clinical research should also seek to better understand the potential effect of vaccines on gastrointestinal flora.
In addition to funders already actively investing in vaccines, working with cystic fibrosis advocacy groups may increase interest in funding vaccine development, and expand awareness amongst patients of the possibility of participating in research that contributes to vaccine development.
Secondary recommendations
Alternative treatments should be explored for P. aeruginosa infection. For example, passive immunisation should be investigated for hospital-acquired P. aeruginosa infections. However, this is a difficult and expensive alternative. Many of the challenges of vaccine development would be shared with the development of monoclonal antibodies, such as the need for good animal models. The benefits of a passive immunisation approach are that it is effective independent of the status of a patient’s adaptive immune system, as antibodies are already formed. Since many patients with P. aeruginosa infections are immunocompromised and may not mount a strong immune response to a vaccine, this is a particularly attractive advantage. Passive immunisation also confers protection immediately which is advantageous because it is difficult to predict which patients will require ventilation or otherwise be at high risk of infection. The ability to give monoclonal antibodies at the time of infection obviates the need to prejudge which patients are likely to be at risk and the need to evaluate acceptable risk thresholds where vaccination is considered necessary. However, this approach also faces many of the same development challenges as vaccines. It is difficult to predict the effect passive immunisation would have on colonisation and, since the protective effects of passive immunisation might only last a few weeks, the approach may not prevent colonisation of the lungs.
Another secondary recommendation is to better understand the disease burden through better elucidating pathogen-level epidemiology. The burden of disease and regional breakdowns are important for determining vaccination strategy and assessing the cost-effectiveness of different strategies. This, in turn, impacts commercial decisions on whether to invest in vaccine development. There is no single study that presents a global view of the incidence, morbidity and mortality caused by P. aeruginosa infection across all relevant clinical syndromes, including direct mortality (such as from hospital-acquired infections), and attributable mortality from colonisation in lung diseases. There is also no regional breakdown of the burden of P. aeruginosa. It is particularly important to gain a clearer understanding of global disease burden for P. aeruginosa because it is a pathogen where current global estimates may be especially conservative. Further work on epidemiology may be aided by the creation of disease registries for cystic fibrosis, bronchiectasis, and other high-risk conditions since these are populations of interest for vaccination. Registries may also facilitate vaccine research by aiding participant recruitment.