Non-typhoidal Salmonella
In high-income countries, non-typhoidal Salmonella (NTS) causes mostly gastrointestinal disease. Invasive NTS (iNTS), which is endemic in Africa, also causes systemic infection with fever. iNTS accounts for ~2% of all NTS cases but ~50% of all NTS mortality. NTS has a high incidence (~150 million cases per year) and mortality (~120,000 deaths per year). Vaccine development has focused almost exclusively on iNTS and the development of a protective vaccine should technically be feasible. Uptake of an iNTS vaccine in low-income countries would be likely, especially in endemic regions in Africa, but would not have a significant market outside of endemic regions. In high-income countries a vaccine would only be used as travellers´ vaccine.
Non-typhoidal Salmonella falls into a cluster of pathogens for which the priority is to bring a vaccine to market. The primary recommendation is to accelerate clinical development. The secondary recommendations are to better understand the burden, epidemiology and transmission of iNTS and to incentivise the development of multi-pathogen/ combination vaccines.
Salmonella are Gram-negative bacteria of the family Enterobacteriaceae but listed separately on the WHO priority pathogen list. There are more than 2,500 NTS serovars with Salmonella Typhimurium and Salmonella Enteritidis accounting for approximately 50% of all human isolates 331.
iNTS is a subset of NTS that causes more serious symptoms and 50% of all NTS mortality and is most common in Africa 332. iNTS strains typically have a distinct genotype and invasive disease is associated with HIV, anemia, malnutrition and malaria 333. Global awareness of the severity of iNTS is low, and one expert states “some people think iNTS is mild but in Africa it’s a very different problem. There’s a lack of awareness in the West, combined with a lack of advocacy in Africa” 28. Experts also believe that until recently iNTS was under-recognised and mis-diagnosed as typhoid fever, as one explains “even clinicians in Africa would have assumed iNTS cases to be typhoid” 28.
iNTS is primarily spread by the faeco-oral route and can rarely be spread through direct person-to-person contact 334,335. Symptoms of non-typhoidal salmonellosis include acute onset of fever, abdominal pain, diarrhoea, nausea and sometimes vomiting 336. Symptoms of invasive non-typhoidal salmonellosis include fever, hepatosplenomegaly, and respiratory symptoms 337. Groups at highest risk for iNTS infection include HIV-infected persons, malaria-infected persons, and malnourished children 337.
Direct health impact
Robust global data on disease burden is not available. Neither the IHME nor WHO provides estimates and a review of the research literature identified few relevant studies. The available data suggests intermediate mortality (~120,000 deaths per year) and low morbidity (~150,000 years lived with disability per year) for NTS infection globally 18. Mortality from iNTS accounts for nearly 50% of these deaths (~55,000 deaths per year) globally 18. The data on morbidity and mortality was taken from an article published by Havelaar et al. in PLoS Medicine in 2015 and only includes infections in non-HIV infected individuals 18. Whilst offering comprehensive and up-to-date epidemiological data on foodborne diseases, experts highlighted that there is some controversy around this data and IHME is currently working on publishing their view on iNTS burden. One expert expresses frustration with the current state of epidemiological data on iNTS infection, stating that “lack of data regarding the epidemiology of the disease is a big impediment” 28. Another noted that “we currently only have very limited data on iNTS disease burden” 28.
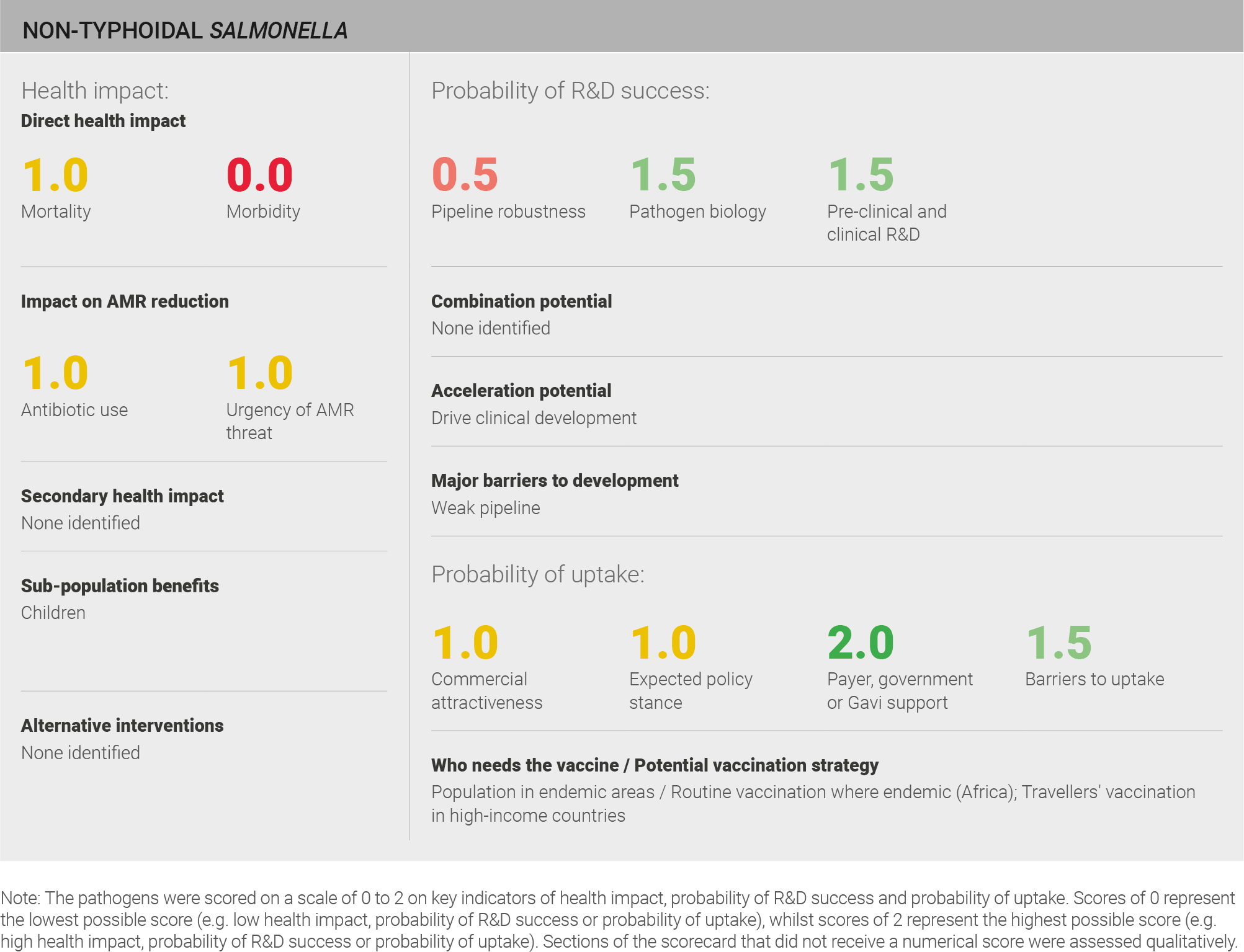
Scoring: Based on the above analysis, mortality was categorised as medium (score of 1 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
The populations likely to benefit from a vaccine for iNTS are infants and young children, particularly in endemic regions, and individuals infected with HIV or malaria.
Antibiotic use
Fluoroquinolones such as ciprofloxacin or levofloxacin are a typical choice for empiric antibiotic treatment, with trimethoprim-sulfamethoxazole, ampicillin or third generation cephalosporins, such as ceftriaxone or cefotaxime , being reasonable alternatives 338.
Scoring: Based on the above analysis, antibiotic use was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~80 million cases treated with a three day course of antibiotics.
Urgency of AMR threat
The WHO lists Salmonella spp. as ‘high’ in its priority list of R&D for new antibiotics 6,339 but it does not appear on the CDC list of biggest threats from AMR 7. Reduced susceptibility and resistance of NTS to fluoroquinolones and third generation cephalosporins (such as ceftriaxone) is increasing, with resistance frequently reported in Asia 338. Ceftriaxone-resistant strains have recently doubled in the United States to approximately 5% 338.
Scoring: Based on the above analysis, the urgency of AMR threat was categorised as medium (score of 1 out of 2).
Pipeline robustness
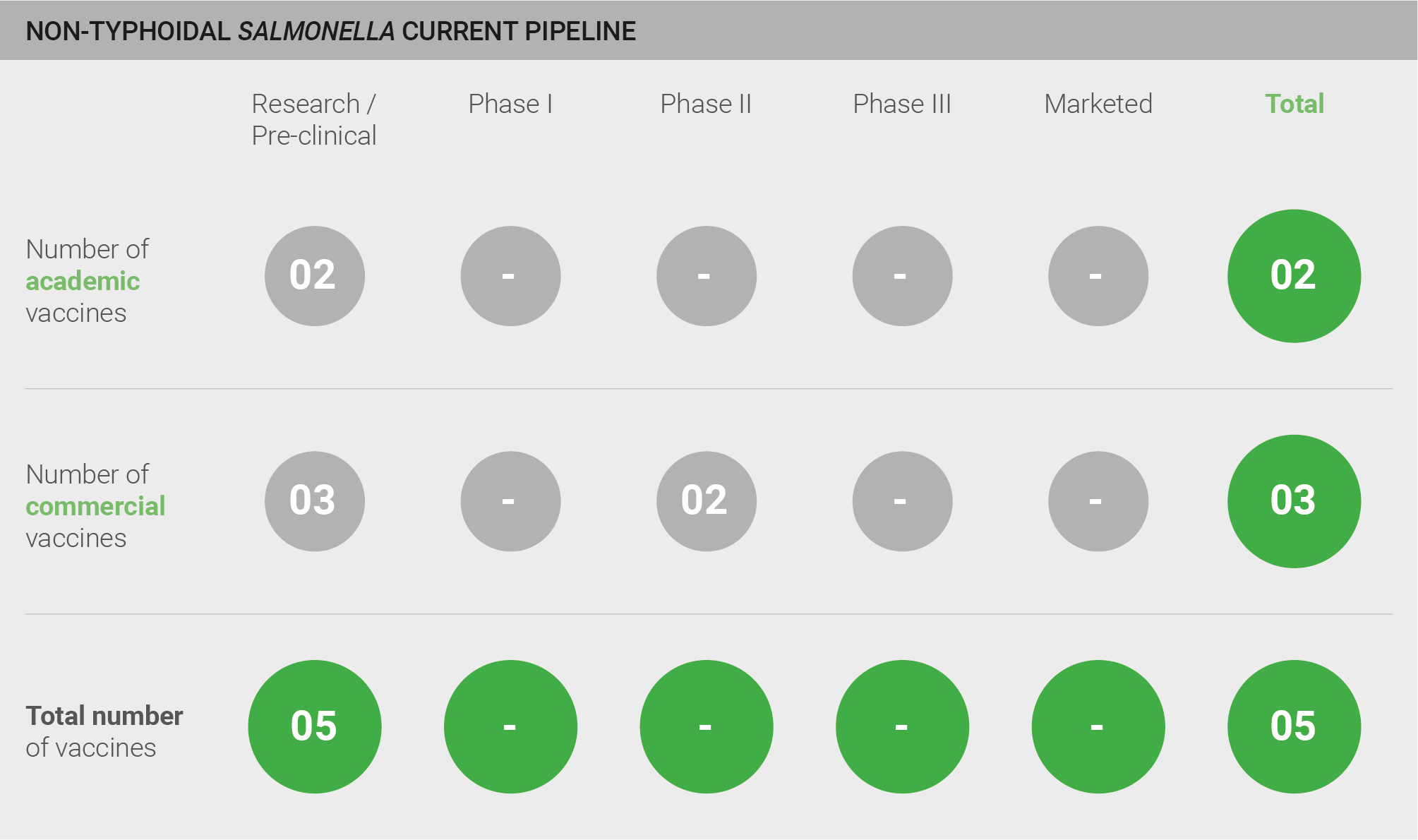
The pipeline for vaccines against iNTS comprises five candidates in pre-clinical development.
Although the pipeline is limited, experts are optimistic about the probability of R&D success, given the biological similarities among Salmonella strains and recent success in developing a conjugated vaccine against S. Typhi that has received prequalification from the WHO 147. One expert summarises the future of vaccine development, saying “I am very confident that vaccine development will be possible given that we know immunogenic targets” 28. Hence, the limited activity in the pipeline does not reflect the feasibility of developing an efficacious vaccine against iNTS, but rather highlights a clear lack of resources and interest to drive pre-clinical and clinical activities forward.
Scoring: Based on the above analysis, the pipeline was categorised as fairly low (score of 0.5 out of 2).
Current pipeline
Pathogen biology
Both antibodies and complement can kill Salmonella in vitro, suggesting that at least partial immunity to iNTS likely exists 331. Patients previously infected with NTS develop serum antibodies that have in vitro bactericidal activity partly by mediating intracellular oxidation 331. Epidemiological studies in sub-Saharan Africa have shown that antibodies against NTS correspond with a decrease in age-related incidence of iNTS disease 331.
Vi antigens have proven to be useful targets for developing a vaccine against the related pathogen, S. Typhi. However, iNTS does not express these antigens and a different vaccine development strategy is needed. Several promising targets and approaches are in pre-clinical development. It is unclear if immunity against these targets will be protective against gastroenteritis and invasive disease, but it is suggested that covering between five and six serovars could protect against the most relevant forms of gastroenteritis and invasive Salmonella worldwide 331.
Approaches in development include: Generalized Modules for Membrane Antigens (GMMA) (providing surface polysaccharides and outer membrane proteins in native conformation), glycoconjugation (linking LPS-derived O polysaccharide to carrier proteins) and protein vaccines (conserved recombinant or purified surface or outer membrane protein antigens (such as flagellin, porins OmpC, F, D)) 331.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly high (score of 1.5 out of 2).
Pre-clinical and clinical R&D
Relatively robust pre-clinical models are available for iNTS. Mice are permissive to S. Typhimurium and S. Enteritidis systemic infection, both of which cause invasive disease without gastritis in mice 331. To produce an NTS enterocolitis infection, mice are pre-treated with streptomycin or other antibiotics prior to bacterial challenge 331. Although these models are more informative than other animal models for some related pathogens (such as S. Typhi/S. Paratyphi), there are noteworthy differences between mouse and human NTS infections.
Correlates of protection have not yet been identified, but data from Malawi show that antibodies to S. Typhimurium (including the surface lipopolysaccharide) are associated with lower risk of NTS bacteraemia, particularly in the first few months of life when maternal antibodies are present 331. The serum bactericidal activity of these antibodies can be measured via an in vitro assay 331. Whilst helpful for clinical trial design, this correlation is not yet adequately characterised to serve as a quasi-correlate of protection (similar to anti-Vi IgG for S. Typhi).
Clinical trials for iNTS are likely feasible, but not all of the necessary elements for clinical development are currently in place. Human challenge models have been considered but have not yet been established due to the early developmental stage of iNTS vaccine candidates 340. According to experts, clinical trial infrastructure in endemic regions is in place and incidence is high, making efficacy trials feasible in Africa 28.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as fairly high (score of 1.5 out of 2).
Expected policy stance
A vaccine against iNTS would be particularly beneficial to children in endemic areas. Therefore, routine vaccination of children in these regions is a likely strategy. A vaccine would also potentially be used as a travellers’ vaccine.
There are reasonable arguments that would suggest policy support for an iNTS vaccine. NTS infection has a greater incidence than S. Typhi infection, and higher mortality than S. Paratyphi infection 31. Experts also cite support from WHO as a reason to anticipate policy support for a vaccine targeting iNTS, with one expert stating that “WHO has a programme to support non-typhoidal vaccines for Salmonella infections” 28.
Scoring: Based on the above analysis, expected policy stance was categorised as medium (score of 1 out of 2).
Payer, government or Gavi support
Payers in high-income countries are unlikely to support vaccination against iNTS due to the low burden of disease in these regions. However, given the high incidence of iNTS in endemic regions, a travellers’ vaccine might be endorsed. Support for an iNTS vaccine is also unlikely in non-endemic middle-income countries, although support for use as a travellers’ vaccine could be possible.
In low-income countries, an iNTS vaccine would align with Gavi’s aim to reduce mortality and invest in diseases where there is a disproportionate impact amongst vulnerable groups. The burden of iNTS is concentrated in African countries, a high proportion of which have Gavi support. A combination vaccine against enteric diseases could further attractiveness of an iNTS vaccine.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as high (score of 2 out of 2).
Barriers to uptake
No new touchpoints or changes to existing clinical practices would be required for iNTS vaccination, as it would be included as part of childhood vaccination programmes or travellers’ vaccination. However, because the burden is predominantly in Africa, the implementation of vaccination programmes may require additional infrastructure for storage and supply chain 28.
Scoring: Based on the above analysis, barriers to uptake was categorised as fairly low (score of 1.5 out of 2).
Commercial attractiveness
The commercial attractiveness of an iNTS vaccine is limited by the likelihood of restricted demand in high- and middle-income countries where it would be used only as a travel vaccine, coupled with uncertainty surrounding the likelihood of Gavi support in low-income countries.
Scoring: Based on the above analysis, commercial attractiveness was categorised as medium (score of 1 out of 2).
Primary recommendation
The primary recommendation is to encourage and accelerate clinical development. Key funders of enteric disease research should be encouraged to support clinical trials for promising vaccine candidates. Although the pipeline appears weak, there are pre-clinical candidates in development that are not listed in official databases. Thus it is stronger than it appears. Additionally, iNTS is a relatively well characterised pathogen, sharing commonalties with S. Typhi, which has demonstrated proof of principle for vaccine development. With greater focus of resources and expertise, candidates could be accelerated through the value chain with relative speed. Opportunities for funders to strategically coordinate efforts facilitating the pooling of resources and funding of later stage trials for iNTS should also be encouraged. Funders should also support technical advancements that enable cheaper vaccine production to ensure uptake in low-income countries. These may include less expensive conjugation methods and GMMA.
Secondary recommendations
A secondary recommendation is to better understand the epidemiology and burden of iNTS. A better understanding of the global and regional burden of iNTS infection is needed to inform policy making and increase the likelihood of Gavi support. Rates of misdiagnosis are high for enteric disease, and treatment may be empiric, so it is important to conduct high-quality studies using laboratory-based diagnostic techniques to provide a more accurate picture of the disease burden 75,341. According to experts, efforts at IHME are currently underway to establish a better fact base on disease burden of iNTS 28, an important step toward better understanding of the burden of disease.