Klebsiella pneumoniae
Klebsiella pneumoniae (K. pneumoniae) primarily causes hospital-acquired infections, commonly presenting as pneumonia. The incidence of K. pneumoniae infection is high compared to other hospital-acquired infections. While there is a high urgency of AMR threat and moderate mortality, morbidity is low. Despite some understanding of pathogen biology the probability of R&D success is low in part due to challenges to identifying conserved antigens. Previous clinical trials have been unsuccessful and it is difficult to identify a target population for the vaccine. Uptake of a vaccine would faces hurdles due to difficulty identifying well circumscribed populations at high risk of infection.
K. pneumoniae falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to better understand the burden, epidemiology and transmission of the pathogen. The secondary recommendations are to explore alternative treatment and prevention strategies and to support pre-clinical research.
K. pneumoniae is a Gram-negative bacterium found in the normal flora of the human mouth and intestine that primarily causes hospital-acquired infections, but can also cause community-acquired infections in immunocompromised patients 242. Although K. pneumoniae is part of the Enterobacteriaceae family, it has been considered separately in this assessment because of its high incidence relative to other members of the Enterobacteriaceae family. It is transmitted on medical equipment, on the hands of healthcare workers, or from environmental reservoirs 243. Common clinical presentations and accompanying symptoms of K. pneumoniae infection include:
- Pneumonia: fever, cough, increased sputum production, pleuritic chest pain, dyspnoea, tachypnoea, crackles on physical examination.
- Urinary tract infection (UTI): frequency, dysuria, malaise, fever, loin pain
- Liver abscess: fever, right upper quadrant abdominal pain, chills
Less common presentations include spontaneous bacterial peritonitis, endophthalmitis, skin and soft tissue infections, and brain abscess.
Populations at greatest risk of K. pneumoniae infection are immunocompromised individuals, including those with diabetes, chronic lung conditions, HIV-positive individuals, and hospitalised patients. Patients who contract K. pneumoniae pneumonia may be on ventilator support, immunocompromised or have chronic airways disease e.g. COPD 242. Those who contract K. pneumoniae UTIs may have catheters 242. Hospital-acquired K. pneumoniae infections occur worldwide, but incidence of community-acquired infection varies by country. For example, Taiwan and South Africa have higher incidence of community-acquired pneumonia caused by K. pneumonia than other countries 242.
Direct health impact
Global data on disease burden is not available from the IHME, WHO or in the research literature 31,32. The global burden of UTIs and lower respiratory tract infections (LRTIs) including pneumonia from all causes are available from the IHME 31. A review of the research literature suggests that K. pneumoniae is responsible for ~7% of UTIs 82 and ~4% of LRTIs 33. Given the lack of direct data on the burden of K. pneumoniae, it is challenging to assess the global burden precisely with confidence. A full methodology for this assessment can be found in the appendix.
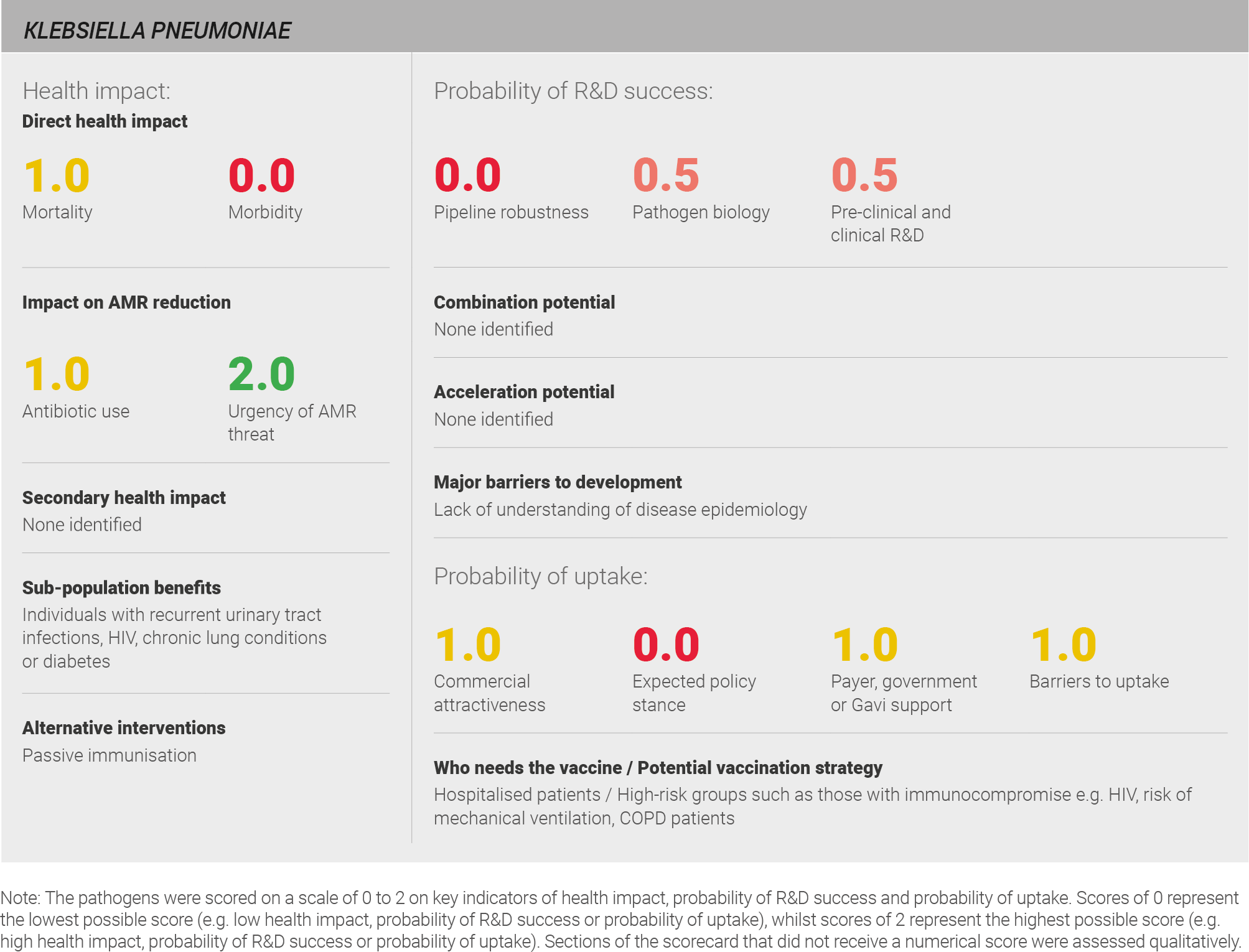
Scoring: Based on the above analysis, mortality was categorised as medium (score of 1 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
A vaccine would particularly benefit immunocompromised individuals, hospitalised patients (including intensive care patients and patients with invasive devices, including urinary catheters 244), the elderly 245, and individuals with chronic conditions (including chronic lung conditions, chronic liver disease, and dialysis patients 245).
Antibiotic use
Typical antibiotic treatment courses for LRTIs and UTIs are approximately one week in duration. An international panel of experts, convened by the Infectious Diseases Society of America (IDSA) in collaboration with the European Society for Microbiology and Infectious Diseases (ESCMID), suggests nitrofurantoin is an appropriate treatment for uncomplicated UTIs 246.
Other appropriate treatments include trimethoprim and beta-lactam/beta-lactamase combinations 118. Treatment for lower respiratory tract infection depends upon hospitalisation status and patient exposure to antibiotics. For hospitalised patients with no risk-factors for drug resistance, piperacillin-tazobactam or cefepime may be used 96. Given the high incidence of UTIs, K. pneumoniae associated antibiotic use is primarily driven by UTIs.
Scoring: Based on the above analysis, antibiotic usage was categorised as medium (score of 1 out of 2). This estimate is based on an annual incidence of ~25 million UTIs and ~10 million LRTIs, both treated with a seven day course of antibiotics.
Urgency of AMR threat
Both the WHO and CDC have expressed concern about K. pneumoniae developing AMR. The WHO has listed K. pneumoniae as a member of the Enterobacteriaceae group of pathogens under the ‘critical’ priority pathogens for R&D regarding new antibiotics 6 and the CDC has listed carbapenem-resistant Enterobacteriaceae as an urgent threat in its list of biggest threats from AMR 7. Extended-spectrum beta-lactamase (ESBL) resistant strains have also been listed as a serious threat on the CDC list 7. Both ESBL and carbapenemase-producing Enterobacteriaceae (CPE) K. pneumoniae strains have been reported worldwide 247,248. CPE strains frequently also exhibit resistance to fluoroquinolones and aminoglycosides 249.
Scoring: Based on the above analysis, the urgency of the AMR threat was categorised as high (score of 2 out of 2).
Pipeline robustness
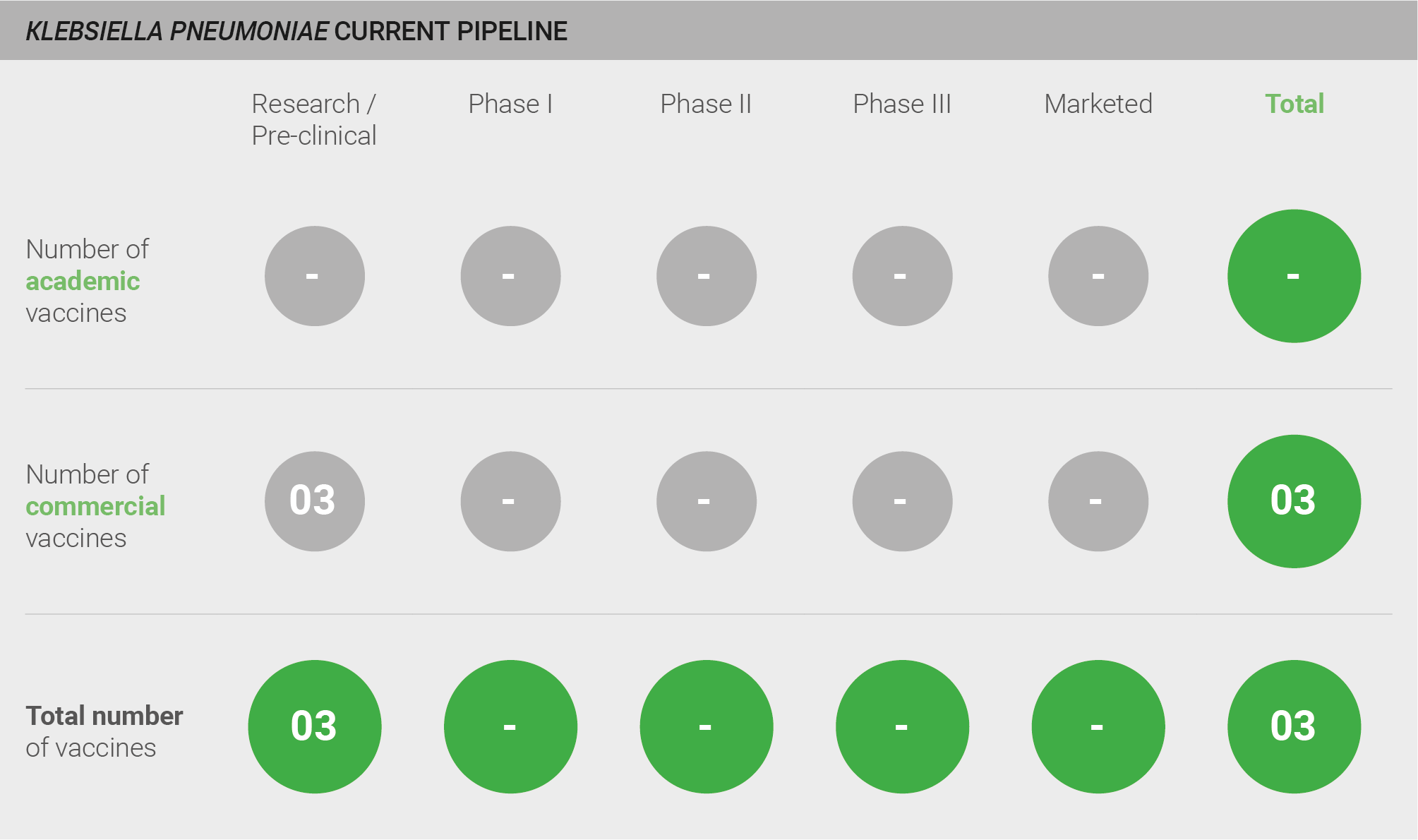
The pipeline is almost empty. There are three vaccine candidates and all are in pre-clinical development 40–42.
Scoring: Based on the above analysis, pipeline robustness was characterised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
Vaccine targets for K. pneumoniae have been explored. O-antigens and K-antigens are potential targets but have significant limitations in K. pneumoniae. These antigens have been studied in detail and to date eight O-antigens and 77 K-antigens have been identified 250. O-antigens do not appear to be useful vaccine targets for K. pneumoniae because they cause adverse toxic reactions in active immunisation 251. K-antigens are immunogenic and non-toxic, but a vaccine would have to include at least 24 major K-types to cover 70% of K. pneumoniae strains 251.
Mannose-resistant type 3 fimbriae are another potential target 250. They are produced by the majority of K. pneumoniae strains and stimulate IL-6, an inflammatory cytokine, demonstrating that they do induce an immune response 99. Targeting mannose-resistant type 3 fimbriae has produced encouraging early-stage results in mouse models in which purified fimbriae from several strains protected mice against K. pneumoniae infection 252.
Overall, however, no single conserved antigen has been identified as a candidate to accelerate for vaccine development.
The development of immunity post-infection appears unlikely and if immunity occurs it would be strain-specific due to the high number of serotypes 251.
Scoring: Based on the analysis above, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
Animal models exist for some K. pneumoniae infection types. Mouse models for acute pneumonia and liver abscess can be used in pre-clinical studies 252,253. However, informative models of chronic processes, especially biofilm formation, are lacking 254. A cutaneous wound model exists but this is not a direct model of chronic lung infection 255.
No vaccines have been approved for K. pneumoniae and none are currently in clinical 40–42. In the past, polyvalent vaccine candidates based on the K-antigen were developed, but they did not progress beyond Phase I trials in humans 251. Therefore, no route to licensure has been established to date. Trial infrastructure would likely be similar to other hospital-acquired infections, and recruitment for efficacy trials may be complicated as there is a need to define target populations 28. Additionally, the emergent and unpredictable nature of emergency hospitalisation makes determining which patients would be eligible for clinical trials difficult.
Scoring: Based on the analysis described above, pre-clinical and clinical R&D for K. pneumoniae was characterised as fairly low (score of 0.5 out of 2).
Expected policy stance
A K. pneumoniae vaccination strategy is difficult to define due to the challenges in targeting those at highest risk of the infection. A strategy could potentially be to vaccinate high-risk groups such as hospitalised patients, and particularly those with compromised immune systems due to HIV infection, diabetes, alcohol dependency or other conditions; and patients with chronic lung diseases; and patients undergoing mechanical ventilation 242.
This pathogen was viewed as an unlikely candidate for vaccination by policy experts who were interviewed, has not been promoted as a vaccine candidate by WHO PDVAC, literature review does not identify evidence of support for vaccination amongst policy makers. The primary factor in favour of vaccination is that incidence of K. pneumoniae is high compared to other hospital-acquired pathogens on the WHO list. However, several factors weigh against vaccination. Identification of a target population is complex and experts do not believe the incidence is sufficient to merit a routine strategy. One expert comments “who would you vaccinate? Gastro-oesophageal reflux disease patients? All people? Elderly? The load is too low” 28.
Scoring: Based on the analysis described above, expected policy stance was categorised as low (score of 0 out of 2).
Payer, government, or Gavi support
Payers and governments in high- and middle-income countries may consider a K. pneumoniae vaccine cost-effective if high-risk target groups could be easily identified. However, as discussed previously, identification of target populations presents a significant challenge. Gavi investment in a K. pneumoniae vaccine is unlikely because direct mortality is low.
Scoring: Based on the analysis described above, payer, government, or Gavi support has been categorised as intermediate (score of 1 out of 2).
Barriers to uptake
Different vaccination touchpoints would be required depending on the target population. Whilst many high-risk groups are in frequent contact with healthcare services, vaccine administration would still need to be incorporated into the existing bundle of interventions received by patients and a new programme would need to be built for every high-risk population identified, as the vaccine may fit into patients’ care pathways in different ways.
New clinical practices would need to be developed for a K. pneumoniae vaccine. To ensure timely integration into the care pathways for all relevant indications, vaccine manufacturers would need to alert guideline setting bodies and specialised societies to any expansion of the indications for vaccination as they are approved, including expansion into other target populations.
Scoring: Based on the analysis described above, barriers to uptake were categorised as intermediate (score of 1 out of 2).
Commercial attractiveness
There is a possible market for a vaccine in high-income countries, coupled with the potential for a high price point. However, there are problems in identifying a specific target population, without which payers cannot accurately evaluate cost-effectiveness, and Gavi support is unlikely given low direct mortality relative to other investment options.
Based on the above analysis, commercial attractiveness was categorised as medium (score of 1 out of 2).
Primary recommendation
The primary recommendation is to gain a better understanding of the disease burden and epidemiology of the pathogen. K. pneumoniae has a higher burden than most other hospital-acquired infections, but more data is needed to help determine whether there are predictable sub-populations to target for clinical development and vaccine delivery. There is a need for research studies that assess the comparative incidence and burden of disease across clinical syndromes to determine which clinical syndromes and which care environments (e.g. intensive care patients, ventilated patients, COPD patients) are most closely associated with K. pneumoniae infection and to identify risk factors that are most closely linked to K. pneumoniae infection.
Additionally, there is a need to more precisely determine burden estimates. There are no estimates of K. pneumoniae burden produced by IHME or WHO global burden studies, and the current understanding of the disease burden in high-income countries is incomplete, and in low-income countries is poor.
Secondary recommendations
Consideration of alternatives for the prevention of K. pneumoniae infection is required. An alternative in patients who deteriorating and at risk of intensive care admission or mechanical ventilation is the use of monoclonal antibodies. The advantage of monoclonal antibodies in this target population is that if a procedure needed to be carried out urgently, monoclonal antibodies would provide rapid protection. However, monoclonal antibodies would not provide the sustained protection required in some populations, such as those requiring longer-term mechanical.