Helicobacter pylori
Helicobacter pylori (H. pylori) is a high-incidence, low-virulence pathogen which colonises >50% of the world’s population. Direct mortality from H. pylori is low and colonisation is most often asymptomatic. However, H. pylori is the most common cause of peptic ulcers and is an important risk factor for gastric cancer. AMR is a growing concern although first-line treatment is effective in the majority of cases. The most advanced vaccine candidate is in Phase I clinical trials and significant barriers remain to successful R&D including: the absence of natural immunity, a lack of suitable vaccine targets and the difficulty in demonstrating efficacy without immunologic correlates. Global uptake of a vaccine is unlikely, but interest may be high in regions where gastric cancer is common.
H. pylori falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to better understand the burden, epidemiology and transmission of the pathogen. The secondary recommendations are to explore alternative treatment and prevention strategies and to support pre-clinical research.
H. pylori is a Gram-negative bacterium that has co-evolved with humans for approximately 60,000 years 215 and is the most common chronic bacterial infection in humans 216. H. pylori commonly colonises the stomach, but other sites are also occasionally colonised 215. It is spread person-to-person in saliva and by faecal contamination of food or water 217.
H. pylori colonisation is most often asymptomatic. However, H. pylori causes many cases of atrophic gastritis 218, peptic ulcer disease 219, and is a risk factor for gastric cancer 220. Symptoms vary depending on the manifestation, but can include epigastric pain, bloating, lack of appetite, nausea, tar-coloured stools in patients with gastritis and peptic ulcer disease, and indigestion, bloating, heartburn, nausea, and abdominal pain in patients with gastric cancer.
Patients at high risk include pregnant women, amongst whom complications of H. pylori may include hyperemesis gravidarum, severe nausea and vomiting 221,222.
H. pylori has a global distribution but prevalence varies greatly by location and development status. Prevalence has been estimated at 51% in low-income countries versus 35% in high-income countries 223. In one systematic review and meta-analysis of 410,879 participants from 73 countries, the highest prevalence was estimated to be in Latin America and the Caribbean (59%) and the lowest in North America (26%) 223. By nation, the highest prevalence was in Nigeria (90%) 223. In a separate systematic review and meta-analysis from 62 countries, the prevalence was estimated to fall between 19% in Switzerland and 88% in Nigeria 214.
Direct health impact
Robust global data on disease burden is not available, and neither WHO nor IHME reports H. pylori asscoiated disease. However, a review of the literature suggests that H. pylori causes significant disease burden. Globally, it is responsible for the majority of peptic ulcer disease and gastric cancer: 70% of gastric ulcers 219 and an estimated 78% of gastric cancer cases 220 are associated with H. pylori. The International Agency for Research on Cancer (IARC) classified H. pylori as a Group I carcinogen in 1994, and confirmed this classification again in 2009 220. An expert states, “it is not disputed that H. pylori causes gastric cancer” 28.
Whilst recent systematic reviews and meta-analyses exist estimating prevalence 214,223, there is a lack of robust data at the global level estimating H. pylori mortality and morbidity by cause. However, robust global data exists for peptic ulcer disease and gastric cancer and estimates for the percentage caused by H. pylori were found in the literature. These estimates are likely to be less precise than the IHME estimates for other diseases. A full description of the methodology used to arrive at the estimates can be found in the appendix.
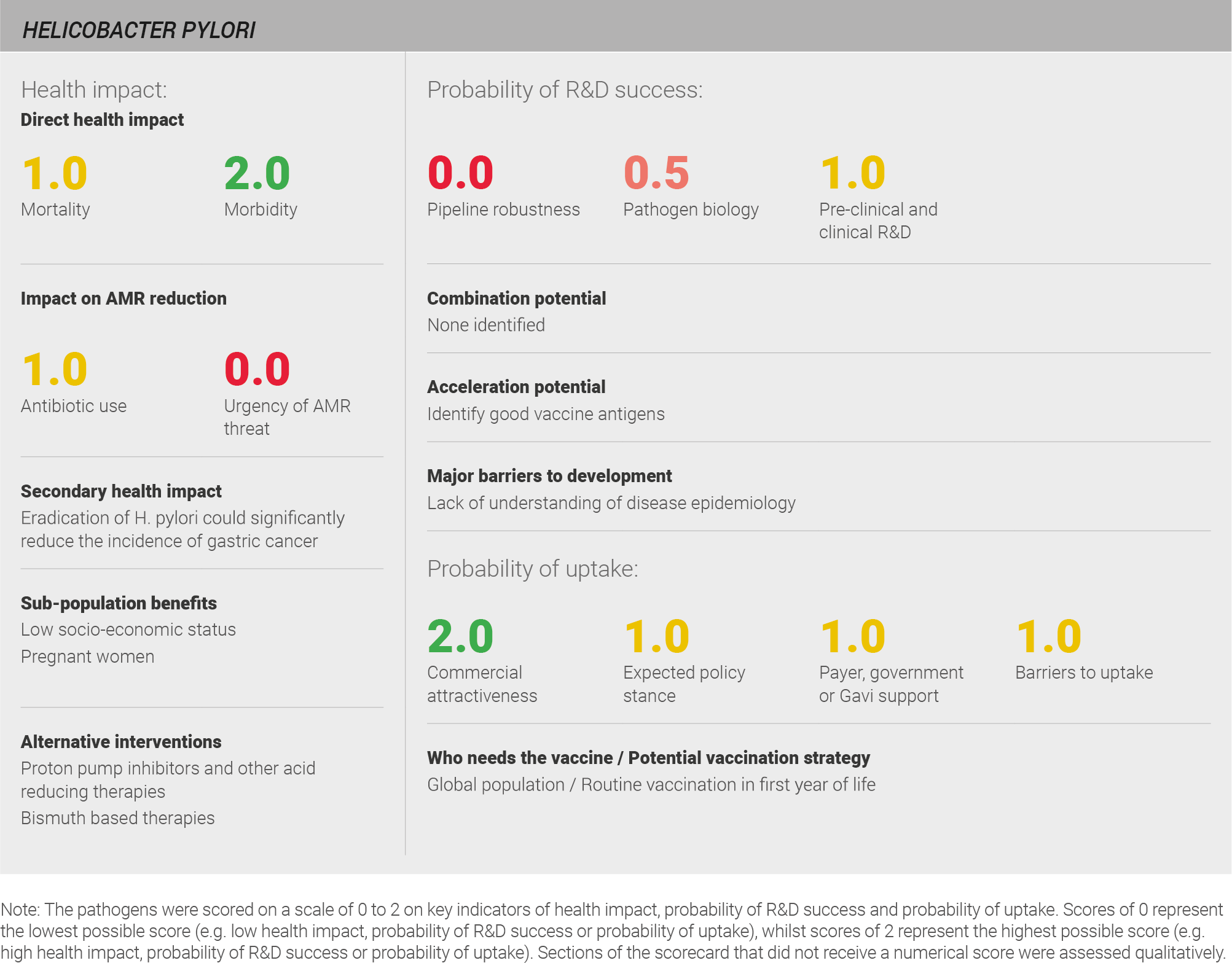
Scoring: Based on the above analysis, mortality was categorised as medium (score of 1 out of 2) and morbidity was categorised as high (score of 2 out of 2).
Secondary health impact
A significant reduction of H. pylori infection would likely be a driver in the reduction of gastric cancer incidence 220.
Sub-population benefits
A vaccine against H. pylori will particularly benefit individuals of low socio-economic status and pregnant women, who are at risk of complications from H. pylori infection 222,224.
Antibiotic use
Many antibiotic regimens have been evaluated for the treatment of H. pylori but few have achieved high pathogen clearance rates in individuals 225. A typical treatment course for H. pylori may be two weeks. In geographical regions where clarithromycin-resistance is known to be low (<15%) and the patient has no history of macrolide exposure, the American College of Gastroenterology (ACG) advises the use of proton pump inhibitors (PPI), clarithromycin, and amoxicillin or metronidazole as a first-line therapy 226. Bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, and concomitant therapy, consisting of a PPI, clarithromycin, amoxicillin and a nitroimidazole, are also recommended options 226.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score of 0 out of 2). This estimate is based on an annual incidence of ~ seven million peptic ulcer disease cases treated with a two week course of antibiotics
Urgency of AMR threat
The WHO has expressed concern about the development of AMR and has listed clarithromycin-resistant H. pylori as a ‘high’ priority pathogen for R&D regarding new antibiotics 6. However, it is not listed on the CDC watch list of most significant threats from AMR 7. Experts have mixed views about the level of threat posed by resistant H. pylori; an expert who is concerned about the risk states “[AMR in H. Pylori is] a problem across countries and the fear is that it will continue to increase” 28. However, another expert disagrees, saying “why is H. pylori on the list? This does not make sense. I see no link between H. pylori and AMR threat” 28.
Resistance rates are increasing in H. pylori 227. Resistance to clarithromycin is developing rapidly in regions where H. pylori seropositivity is high 227. Clarithromycin resistance is particularly prevalent in China, where it is estimated to affect half of cases 227. Less longitudinal data is available for other antibiotics but metronidazole resistance is confirmed to be increasing in many countries 227
However, resistance to tetracycline and amoxicillin, which are both included in American College of Gastroenterology recommended first-line treatment courses, are very low <2% 227, and treatment with first-line agents is still successful in the majority of cases (~85% of cases in the United States 228 and a lower number in Europe). An expert explains “at the moment you can generally control the infection in most people” 28.
Scoring: Based on the above analysis, the urgency of AMR threat for H. pylori was categorised as low (score of 0 out of 2).
Pipeline robustness
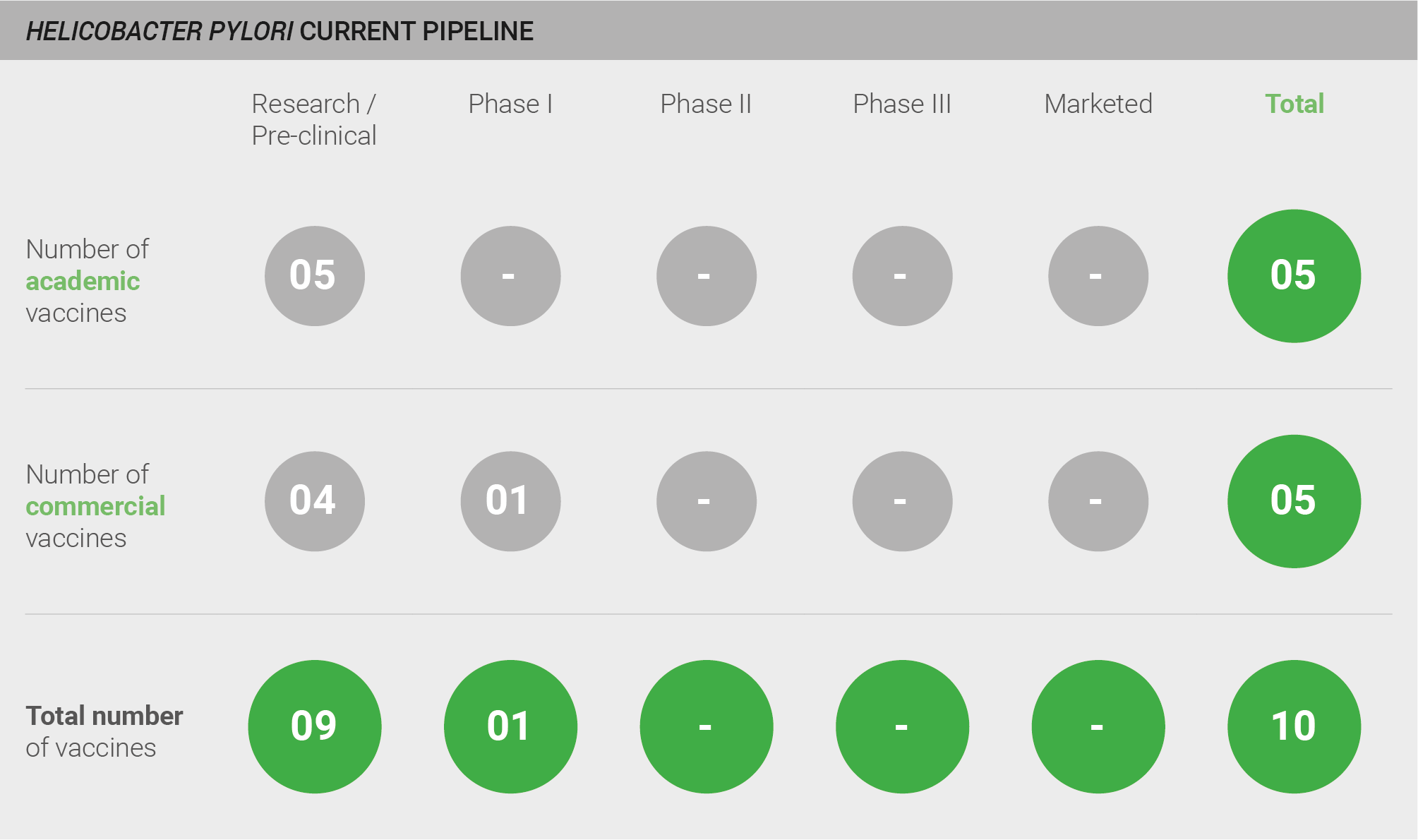
The H. pylori pipeline is weak, with a total of 10 vaccines in development. Nine are in pre-clinical development, and one is in Phase I.
Scoring: Based on the analysis described above, the pipeline for H. pylori was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
It is not yet clear if natural immunity exists; however, if it exists, it is only partially effective.
There is evidence from observational research that some children are able to spontaneously clear H. pylori 229. However, this may be attributable to exposure to antibiotic treatment for conditions other than H. pylori infection and is not necessarily evidence of natural immunity 230. The best evidence for natural immune-mediated protection against H. pylori infection derives from a clinical trial where volunteers were exposed to H. pylori following experimental exposure to a live vaccine 231. The vaccine was not effective but a minority of participants cleared the H. pylori challenge via a mechanism associated with a T-helper cell response 231. Antibody-mediated protective immunity has not been demonstrated 230.
H. pylori typically colonises a physiologically unique environment, which is both acidic and mucosal. It is still unclear whether this requires a specifically adapted immune response for effective protection. The unique colonisation environment also presents challenges for vaccine development, as one expert explains “[a vaccine] could be of interest but would require mucosal protection through oral tablet or intramuscular injection” 28.
Vaccination in mice using a range of antigens can modestly reduce H. pylori colonisation. However, translation into success in clinical trials has not been shown so far, with the exception of one trial in China 230. The trial was a Phase III, single-centre, double-blind, placebo-controlled, randomised trial of an oral recombinant H. pylori vaccine conducted in Ganyu County, Jiangsu Province, China 232 that enrolled 4464 healthy children aged 6-15 years without past or present H. pylori infection. The trial was sponsored by Jiangsu Province Centers for Disease Control and Prevention in collaboration with National Institutes for Food and Drug Control, China, Kangwei biological technology Co., Ltd (renamed Wuhu Kangwei biological technology Co., Ltd. in 2011) and Third Military Medical University. The trial was conducted from 2005-2007 but results were not published until 2015. The vaccine tested in this trial showed evidence of protection against H. pylori infection (72% efficacy in the first year, falling to 55% after three years of follow-up). Experts cite this study as “proof of concept that a vaccine against H. pylori is possible” , but development of the vaccine has been discontinued for unknown reasons Despite the likely feasibility of a vaccine, experts do not regard currently identified targets as promising: one states “there’s nothing published that I would consider a good target” 28.
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
No correlates of protection have been identified that can facilitate pre-clinical research. A number of animal models are available and have some value for initial screening of vaccine candidates. However, vaccines shown to have some efficacy in mice have not been effective in clinical trials 230. The lack of translatability may reflect insufficient protection in mouse models before progressing to clinical trials and may also reflect poor translatability of protective immunity between mouse models and humans. Lack of investment in pre-clinical research is a major barrier to R&D 28. Experts state that “it’s pretty tough to get money” 28 and “[the] biggest obstacle is investment” 28.
The lack of identified correlates of protection also constrains clinical research, as there is limited information available to help simplify study read-outs. Some experts believe this is particularly problematic for H. pylori vaccine trials because the outcomes of interest, peptic ulcer disease and cancer, occur long after infection. An expert explains “symptom latency is so far in the future you would need a surrogate or correlate of protection to prove efficacy” 28. However, other experts disagree that this is any more problematic for H. pylori than for other pathogens, with one stating “you don’t have to prove correlates of protection against ulcers or cancer because it’s so well ingrained that there’s a cause…the vaccine only has to prove eradication of infection. If you can do that, your clinical path should be quite straightforward” 28. Controlled human infection trials are possible for H. pylori. A challenge strain has been developed and has proven valuable for early clinical trials 233.
The target population for an H. pylori vaccine is not yet clearly defined and could vary depending on specific vaccine technologies. A vaccine could be given prophylactically to prevent infection, or therapeutically to decrease or eliminate colonisation and prevent complications. Prophylactic vaccines would have to be administered in early life, as most adults who are infected became infected in childhood. In contrast, a therapeutic vaccine could be given at almost any age, but research would need to establish lead times between infection and complications.
Other vaccines, primarily targeting the urease antigen 230 have reached clinical trials but have not been successful. Currently, there is one candidate vaccine in clinical trials. ImevaX’s IMX 101 is currently being investigated in a Phase I trial 234. The trial is a multi-center, randomised, double-blind and adjuvant-controlled study to evaluate the safety, tolerability, and efficacy of IMX101 in H. pylori-negative and H. pylori-infected healthy volunteers. The study was initiated in 2017. The primary outcome is safety and tolerability, and the secondary outcome is determination of immune responses to the vaccine 234. IMX 101 comprises an H. pylori antigen (γ-glutamyltranspeptidase (GGT)), an outer membrane protein and a muscosal adjuvant. GGT has been chosen because of its potent immunosuppressive activity which is an important part of H. pylori’s immune evasion. The vaccine aims to neutralise this defence mechanism, facilitating a more effective immune response against other components of a vaccine 230.
Scoring: Based on the analysis described above, pre-clinical and clinical R&D was categorised as medium (score of 1 out of 2).
Expected policy stance
The ideal vaccination strategy has not yet been determined. A prophylactic vaccine would likely require routine vaccination in young infants prior to exposure. A therapeutic vaccine could target older age groups but would require research to help develop a strategy and vaccination schedule best suited to preventing complications.
Experts suggest that policy support may be generated by interest in preventing cancer, rather than the risk of AMR. According to one expert “the real reason to vaccinate is stomach cancer not antibiotic use” 28. The risk of gastric cancer is not always reduced by treatment 235. In particular, treatment with proton pump inhibitors may increase gastric cancer risk, although this link remains uncertain 236,237. The IARC note: “Theoretically, active immunization of young children against H. pylori would be ideal to prevent infection and its chronic consequences, including peptic ulcer disease and gastric cancer” 220, suggesting that there may be WHO support for a vaccine on these grounds. However, to date there is no specific advocacy for the development of a vaccine from policy bodies. This may be because there is a general perception that H. pylori has a good range of antibiotic treatment options and is not a serious disease, as one expert states “the disease is treatable, hence a vaccine is not interesting” 28 and another agrees, saying “I would not lose a lot of sleep on H. pylori” 28.
Scoring: Based on the analysis described above expected policy stance was categorised as medium (score of 1 out of 2).
Payer, government, or Gavi support
In high- and middle-income countries, a routine, prophylactic vaccine may be cost-effective 230.
The cost-effectiveness of prophylactic vaccination strategy targeting infants was estimated to be ~$4000/QALY (quality adjusted life year) in the United States and, thus, cost-effective 238. In areas of higher incidence, the cost-effectiveness could be expected to be larger.
Gastric cancer is likely to be a more significant cost lever than peptic ulcer disease. Whilst H. pylori prevalence is highest in Africa, the burden of gastric cancer is highest in Japan, China and Korea 214. This may be related to the predominant H. pylori strains in these regions 214. Because of the high burden of complications in Asia, it may be the region with greatest need for a vaccine and interest in a vaccine may be highest in areas of high gastric cancer risk such as Japan and Korea, and some large middle-income countries such as China and Russia. As one expert notes “[uptake] would depend on the risk of stomach cancer in a country or population so Japan might use this first” 28
Direct mortality from H. pylori infection is low, so investment from Gavi in low-income countries is unlikely. However, the pathogen disproportionately impacts those in low socioeconomic groups, which is something considered in Gavi decision-making frameworks 223,229.
Scoring: Based on the analysis described above, payer, government, or Gavi support has been categorised as medium (score of 1 out of 2).
Barriers to uptake
Based on cost-effectiveness estimations and the increasing prevalence of infection with age, a prophylactic vaccine would need to be administered early in life. As one expert states, “[we] would need to vaccinate very early in life and have to add to existing routine immunisation schedule” 28. No new touchpoints would be required for addition to the routine immunisation schedule. For a therapeutic vaccine, however, target age groups, or other defined populations, may need to be identified. Whilst a therapeutic vaccine could be integrated into existing adult touchpoints, a new touchpoint might be required depending on the target group selected.
Given the low perceived threat of H. pylori, there may be issues of patient acceptance of a vaccine and a strong patient education programme would be required. The particularly low perceived threat of H. pylori would also affect clinical practices, as the benefits of vaccination would have to be clearly set out to guideline setting bodies.
Scoring: Based on the analysis described above, barriers to uptake were categorised as medium (score of 1 out of 2).
Commercial attractiveness
Commercial attractiveness was categorised as high, reflecting a potentially large target population in high- and middle-income countries. However, likelihood of approval for use in a larger population will likely be difficult, as reflected in the sections above.
Scoring: Based on the analysis described above, commercial attractiveness was categorised as high (score of 2 out of 2).
Primary recommendation
The primary recommendation is to collect more data on H. pylori. A better understanding of the contribution of H. pylori to AMR is needed, including clarification of the pace at which the pathogen is developing resistance to different antibiotics and an understanding of how antibiotic use for H. pylori is contributing to the development of resistance in other pathogens. In particular, there is a need to document any cases that are resistant to all treatment.
There is also a significant need to better understand the link between H. pylori and cancer. Currently, there is uncertainty about whether it is H. pylori colonisation or manifest disease which increases the risk of cancer. It is also not yet known whether effective treatment of H. pylori alone would manage the associated cancer risk. There is some evidence that long-term exposure to proton pump inhibitors increases the incidence of H. pylori-associated gastric cancer 236,237. The association between H. pylori-associated cancer risk and treatments for H. pylori and for related gastric symptoms and conditions requires better understanding.
Secondary recommendations
Alternative approaches to manage H. pylori infection should be explored, including the addition of bismuth to conventional triple therapy 239,240, which can achieve high levels of H. pylori eradication. In order to determine the best treatment options for individual infections, particularly where first-line therapies have failed, better diagnostic tests for the detection of drug resistance in H. pylori should be explored 241. Existing methods, including agar culture, agar dilution, disk diffusion and the Etest, all have specific drawbacks and genomic techniques offer a promising potential alternative to these methods. Further, the use of stool samples, as opposed to gastric biopsy, would reduce the need for an invasive procedure to detect antimicrobial resistance and warrants further exploration 241.
Pre-clinical research to better understand H. pylori’s immune evasion mechanisms and how to develop effective vaccines against pathogens that colonise the acidic, mucosal environment of the stomach, in general, would increase the likelihood of developing a successful vaccine.