Haemophilus influenzae
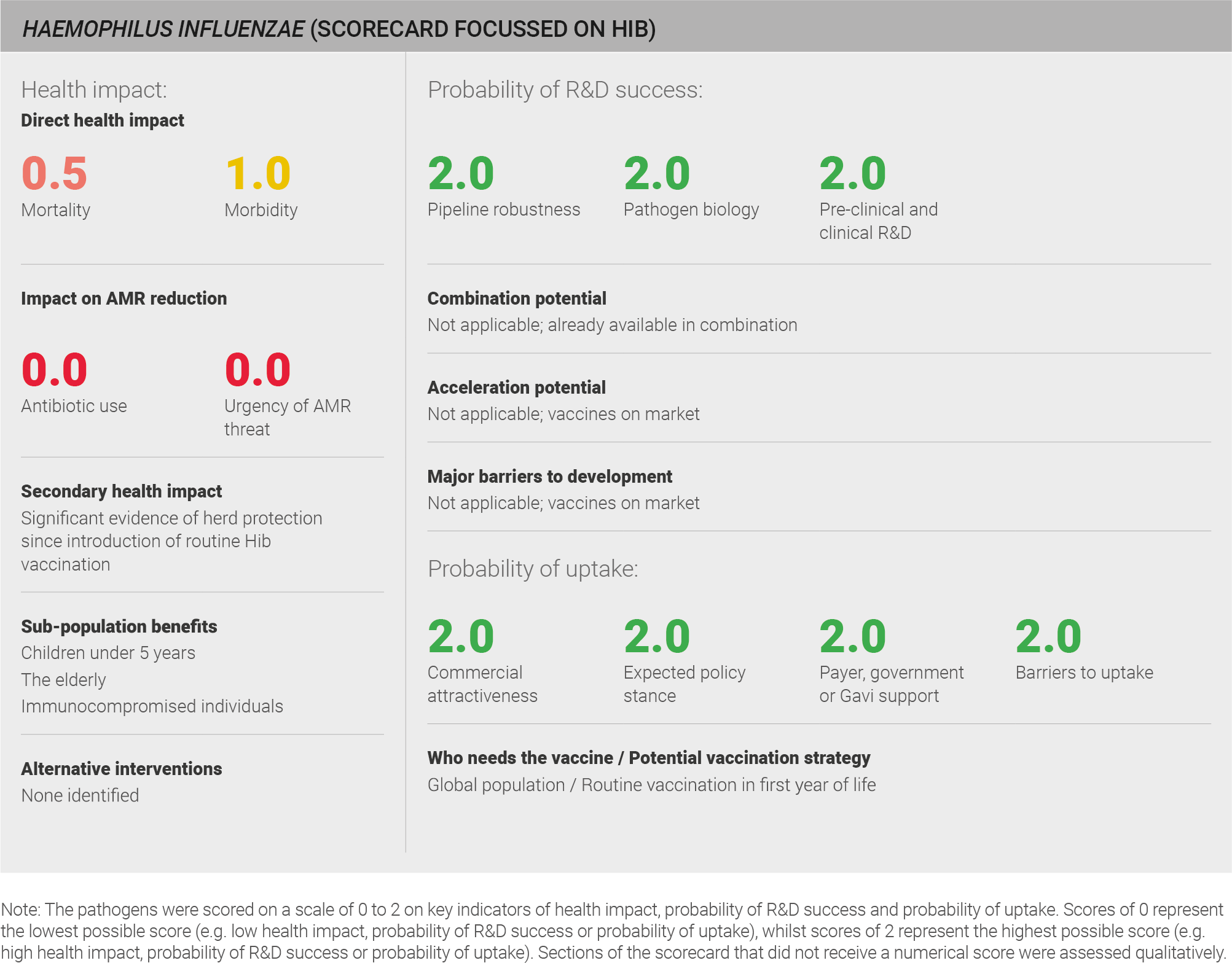
Haemophilus influenzae (H. influenzae) causes pneumonia, meningitis and otitis media, and has a disproportionate effect on children under five. H. influenzae serotype b (Hib) is responsible for 95% of all invasive disease caused by H. influenzae in non-immunised populations. As a result, this report primarily focused on Hib. Effective, conjugated Hib vaccines have been on the market since the 1990s and have strong uptake, with estimated global coverage of ~70%. Current concern surrounding AMR is low as several antibiotics remain effective and vaccination programmes have been shown to reduce AMR. Although uptake is relatively high globally (~70%), continued efforts can be made to further expand coverage of the Hib vaccine.
H. influenzae falls into a cluster of pathogens for which the priority is to increase vaccine uptake. The primary recommendation is to drive coverage and equity. The secondary recommendation is to better understand the disease burden, epidemiology, and transmission, particularly of non-Hib strains.
H. influenzae is a Gram-negative bacterium that is present as a commensal organism in the nasopharynx of most healthy adults 185. However, it can spread to cause both systemic and respiratory tract infection 185. H. influenzae is divided into typeable and non-typeable forms based on the presence or absence of encapsulation by a polysaccharide capsule. There are six typeable serotypes of H. influenzae 186.
H. influenzae type b (Hib), a typeable form, is the most pathogenic to humans and is also the most virulent 178. Hib is responsible for ~95% of all invasiveH. influenzaeinfections in unimmunised populations 178. Non-typeable strains are rare causes of serious infection among children but are a common cause of ear infections in children and bronchitis in adults 187.
H. influenzae is spread through airborne droplets and direct contact with respiratory secretions 184 and most commonly causes pneumonia 188 but can also cause meningitis, epiglottitis, septic arthritis, cellulitis, otitis media, and purulent pericarditis 189. Symptoms vary depending on the manifestation, but can include the following:
- Pneumonia: high fever, headache, severe aches and pains, lethargy, dry cough 188
- Meningitis: nausea and vomiting, confusion and disorientation, drowsiness or sluggishness, sensitivity to bright light, poor appetite, seizure, coma
- Epiglottitis: sore throat, fever, dyspnoea, dysphagia, drooling 190
- Cellulitis: fever, warm skin, erythema, pain, most often located on the cheek or periorbital region 190
Groups at high risk of H. influenzae infection include children under five years, particularly those aged between four and 18 months, with the exception of very young infants who are protected by the transfer of maternal IgG specific for polyribosyl-ribitoal -phosphate (PRP) across the placenta 191; adults aged 65 or older 192; and those with immune-compromising conditions such as complement deficiency, hypogammaglobulinaemia, sickle cell anaemia, functional asplenia, malignancy, and HIV 184,193.
H. influenzae has a global distribution but prevalence varies by location. Although vaccines are available and widely used in many regions, most cases occur in unvaccinated young children in low-income countries 178.
Direct health impact
The following impact evaluation addresses Hib, as it is the most virulent of the H. influenzae strains and causes both the majority (95%) of H. influenzae-associated invasive disease in the absence of vaccination 179,180 and the majority of H-influenzae-associated deaths 179,180. Global data on disease burden for Hib is available from the IHME. The IHME data comprises mortality and morbidity from Hib meningitis and Hib pneumonia. This data uses a defined methodology and is used in the global health community. The data can therefore be viewed with a reasonable level of confidence. Whilst mortality from Hib meningitis is low, the IHME estimates suggest that Hib meningitis is still a significant cause of morbidity globally. Hib meningitis is estimated to be responsible for approximately 30,000 deaths and 0.25 million years lived with disability in 2016 31. Hib pneumonia is estimated to be responsible for approximately 48,000 deaths and 10,000 years lived with disability in 2016 31. Very limited data for non-Hib H. influenzae exists, and one expert explains “it is difficult to know about the disease burden because there is so little information”28 but it is not thought to significantly contribute to the burden of invasive disease 28.
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2) and morbidity was categorised as medium (score of 1 out of 2).
Secondary health impact
There is evidence of significant herd protectionfor Hib 194. With vaccine coverage of <70%, Hib incidence was reduced dramatically in the Gambia with both vaccinated and unvaccinated children benefitting 195. Humans are the only known reservoir of H. influenzae 186.
Sub-population benefits
Vaccination particularly benefits young children and immunocompromised individuals, the groups at greatest risk of H. influenzae infection.
Antibiotic use
A seven day course of antibiotics is a typical treatment for both meningitis and lower respiratory tract infection (LRTI), including pneumonia196,197. Beta-lactam agents such as amoxicillin or a second- or third-generation cephalosporin are the preferred treatment choice 184. Alternative agents with activity against H. influenzae include fluoroquinolones, macrolides, tetracyclines, and aminoglycosides 184. Antibiotic use associated with Hib is generally driven by LRTI as the incidence of LRTI exceeds that of meningitis.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score of 0 out of 2). This estimate is based on an annual incidence of ~eight million LRTIs and ~400,000 meningitis cases, both treated with a one week course of antibiotics
Urgency of AMR threat
The WHO has expressed concern about the development of AMR in H. influenzae and has listed ampicillin-resistant H. influenzae as a ‘medium’ priority pathogen for R&D regarding new antibiotics 6.
However, it is not included on the CDC’s list of biggest threats from AMR 7. Beta-lactamase–negative, ampicillin-resistant H. influenzae is an emerging problem amongst both Hib and other H. influenzae strains.
Prevalence of resistance in all H. influenzae strains is currently at ~35% in Japan, ~55% in Spain, and ~3% in the United States 184. Resistant strains therefore represent a growing threat, but H. influenzae remains susceptible to ceftriaxone 184.
Studies have shown that use of the Hib vaccine is correlated with a reduction in AMR. One 10-year study showed a 50% decrease in ampicillin-resistance and resistance to other, related antibiotics after universal introduction of the Hib vaccine in 1999 183, and an expert states “vaccines are already playing an important role to reduce AMR in S. pneumoniae and Hib” 28. Some experts expressed surprise that H. influenzae was on the WHO priority list, as they do not regard the AMR risk from the pathogen to be high. Furthermore, given the existence of an effective vaccine experts cite a perception that “not much more [is] needed here” 28.
Scoring: Based on the above analysis, urgency of AMR threat was categorised as low (score of 0 out of 2).
Pipeline robustness
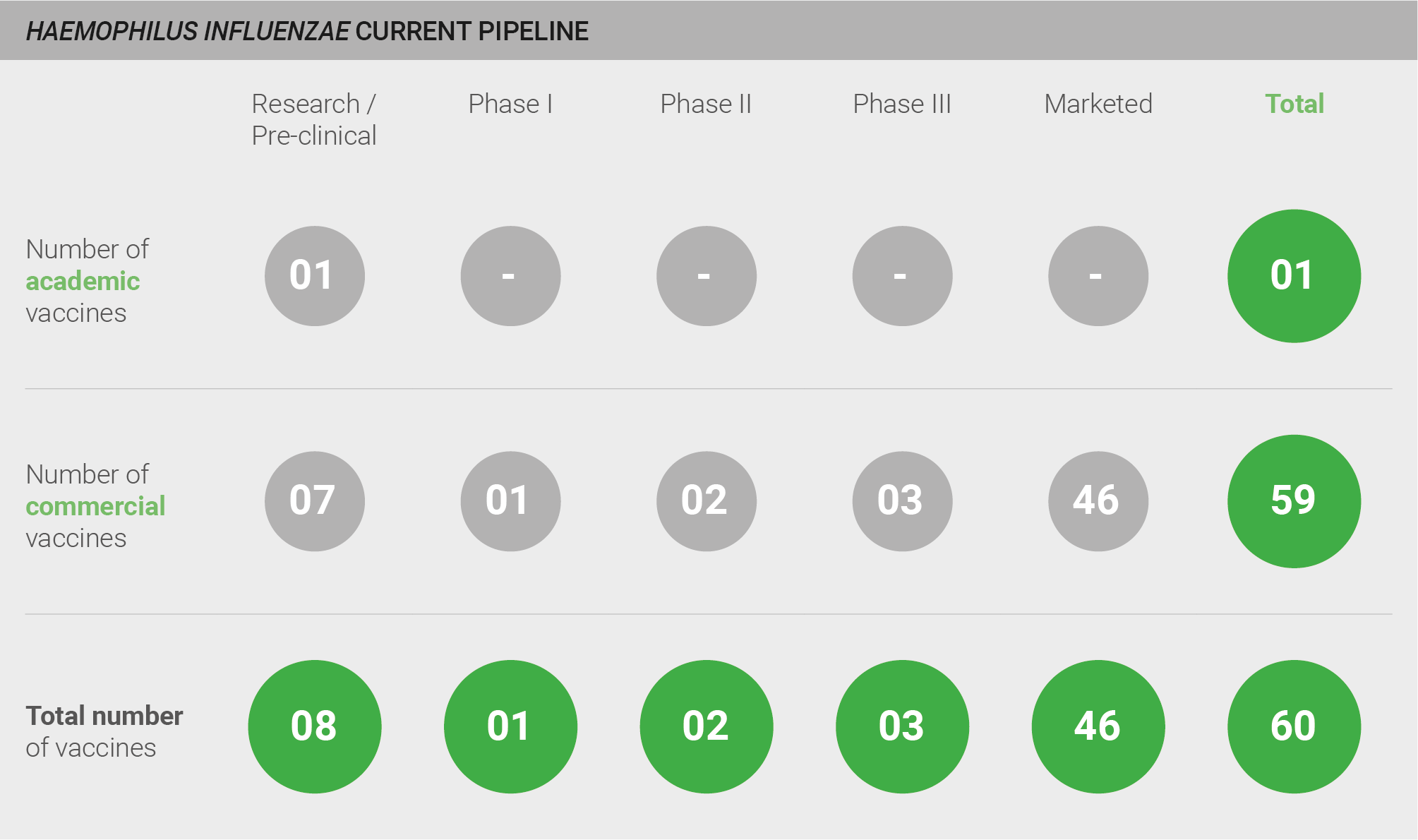
The H. influenzae pipeline is robust, comprising a total of 60 vaccines and including 46 licensed vaccines. Those still in development include eight in pre-clinical studies, one in Phase I, two in Phase II, and three in Phase III. Nearly all of these vaccines – 59 of 60 – are commercially developed. All licensed vaccines and the majority of vaccines in development target Hib. However, GSK is also developing a vaccine against non-typeable H. influenzae 198. Given the focus on Hib, the remainder of the probability of R&D success section addresses Hib vaccines. More detail on the pipeline can be found in the appendix.
Scoring: Based on the above analysis, the pipeline for Hib was categorised as high (score of 2 out of 2).
Current pipeline
Pathogen biology
Since Hib causes the majority of all H. influenzae infections, it has been the focus of most research on pathogen biology for H. influenzae to date. Natural immunity to Hib exists and is well understood.
Strain-specific immunity that is mediated by serum capsular polysaccharide specific IgG antibodies exists and has been known since the 1930s 191. Age-specific profiles of these protective antibodies show a characteristic pattern: high levels of trans-placentally acquired anti-PRP antibodies are present at birth, then fade after birth and have a half-life of approximately 28 days 199. Anti-PRP antibodies reach very low levels by around 6 months of age, but antibody titres rise again during the second year of life 200. This rise in antibody levels is thought to be a response to exposure to Hib in the nasopharynx, or exposure to other organisms with cross-reactive antigens.
Vaccine targets are well-characterised as vaccines against Hib are already on the market. The Hib capsule is formed from repeating polymers of ribosyl and ribitol-phosphate and is called a PRP capsule 191. In vaccines against Hib, PRP is conjugated to carrier proteins to induce a greater immune response. The carrier proteins are involved in T-cell activation and induce immune memory 191.
Scoring: Based on the above analysis, pathogen biology was categorised as high (score of 2 out of 2).
Pre-clinical and clinical R&D
Pre-clinical research benefits from the well-developed base of knowledge for H. influenzae. Anti-PRP antibody titres are known correlates of protection 200. Mouse, rat, guinea-pig and rabbit models are available and have been effectively used to assess the immunogenicity of H. influenzae vaccines 201.
Similarly, correlates of protection are known and used in clinical studies 202: 0.15µg/ml anti-PRP IgG is established as the level needed to give short-term protection from invasive Hib disease 203 and field studies set a threshold of 1µg/ml, one month after completion of the primary vaccination series, as the level required to confer long-term protection from invasive Hib disease 203. The infrastructure needed to run clinical trials is also available.
Licensed vaccines against Hib are efficacious and effective. They are marketed in multivalent combinations. The effectiveness of current vaccines against Hib meningitis is 55% after one dose, 96% after two doses, and 96% after three doses 182. These vaccines are also effective against invasive Hib, showing 59% effectiveness after one dose and 97% after three doses. Insufficient data is available to estimate effectiveness after two doses 182. The efficacy data do highlight the importance of ensuring target populations receive a complete vaccine series as two or more doses are required for high effectiveness 182. Current vaccines are regarded as very safe by the WHO and CDC 204,205 and also shown to be safe and efficacious in multivalent combination vaccines (for example, pentavalent or even hexavalent DTPa-HBV-IPV/Hib).
Licensed vaccines are conjugated polysaccharide vaccines, a proven technology used to develop vaccines against a variety of pathogens. The vaccine is produced in high quantities from several manufacturers worldwide. Combination vaccines with other childhood vaccines are approved and on the market. A typical wholesale cost of a DTPw-HepB-Hib pentavalent vaccine was about 15.40 USD in 2014. Current vaccines do require refrigerated storage; Infanrix (6-valent) has a shelf life of three years and is to be stored in a refrigerator (4°C) 206 and all Hib-containing vaccines should be stored between 2 and 8 °C 203 and liquid vaccines should never be frozen.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as high (score of 2 out of 2).
Expected policy stance
Strong and established policy support for Hib vaccination already exists. The WHO recommends the inclusion of conjugate Hib vaccines in all infant immunisation programmes 203 and has taken this position since 2006. Experts concur that “Haemophilus vaccination programmes should be expanded as much as possible” 28.
Scoring: Based on the above analysis, expected policy stance was characterised as high (score of 2 out of 2).
Likelihood of payer, government, or Gavi support
More than 90% of countries provide the Hib vaccine through routine vaccination schedules 207. By the end of 2017, 191 countries (>95% of WHO member states) had included conjugated Hib vaccines in their immunization programmes 181,203. By the end of 2014, all Gavi-supported countries had introduced the Hib vaccine as part of the pentavalent vaccine 208.
Scoring: Based on the above analysis, likelihood of payer, government, or Gavi support was characterised as high (score of 2 out of 2).
Barriers to uptake
At a national level, uptake of the Hib vaccine is high with 191 countries including Hib in vaccination programmes by the end of 2017 181. Global coverage with three doses is estimated to be ~72% 181 but varies by region 181. The highest coverage is estimated at 91% in the WHO region of the Americas and the lowest estimated at 28% in the WHO Western Pacific Region 181.
Scoring: Based on the above analysis, barriers to uptake was characterised as low (score of 2 out of 2).
Commercial attractiveness
The Hib vaccine is licensed and administration rates are high worldwide.
Scoring: Based on the above analysis, commercial attractiveness was categorised as high (score of 2 out of 2).
Primary recommendation
Although uptake of the Hib vaccine is relatively high globally, continued efforts can be made to further expand coverage. The primary recommendation is to drive coverage and equity. Global uptake of the Hib vaccine is estimated to be ~70% 181. Immunisation is the most important strategy for prevention of Hib infection 209 and uptake should continue to be monitored to identify areas where intervention is needed to drive better coverage. The importance of sustained vaccination against Hib was highlighted during a Hib vaccine shortage in the United States between November 2007 and March 2008 during which outbreaks were reported in Minnesota and Pennsylvania 210.
Secondary recommendation
The secondary recommendation is to better understand disease burden, epidemiology or transmission. Epidemiological data related to non-Hib H. influenzae infections is scarce. Although studies have not comprehensively assessed global burden, estimates based on the literature suggest that there is relatively low mortality 179,180 and incidence 183,211–213 associated with these infections. Better characterisation of the burden would lead to a greater understanding of H. influenzae and would assist in evaluating the case for the development of a vaccine against non-typeable H. influenzae infections. This evaluation should include antibiotic use driven by non-Hib H. influenzae.