Enterobacteriaceae (excl. E. coli & K.pneumoniae)
Bacteria of the family Enterobacteriaceae are a normal part of the gut flora, but in rare cases can cause hospital-acquired infections. Enterobacteriaceae are a family of Gram-negative bacteria that predominantly cause hospital-acquired infections. Enterobacteriaceae family members included included on the WHO priority pathogens list are: K. pneumoniae, E. coli, Enterobacter spp, Serratia spp, Proteus spp, Providencia spp and Morganella spp. E. coli and K. pneumoniae are considered in separately because the higher burden of disease merits individual assessment. Additionally, the Enterobacteriaceae family also includes Salmonella and Shigella, but these are listed as separate pathogens on the WHO priority pathogen list and evaluated separately.
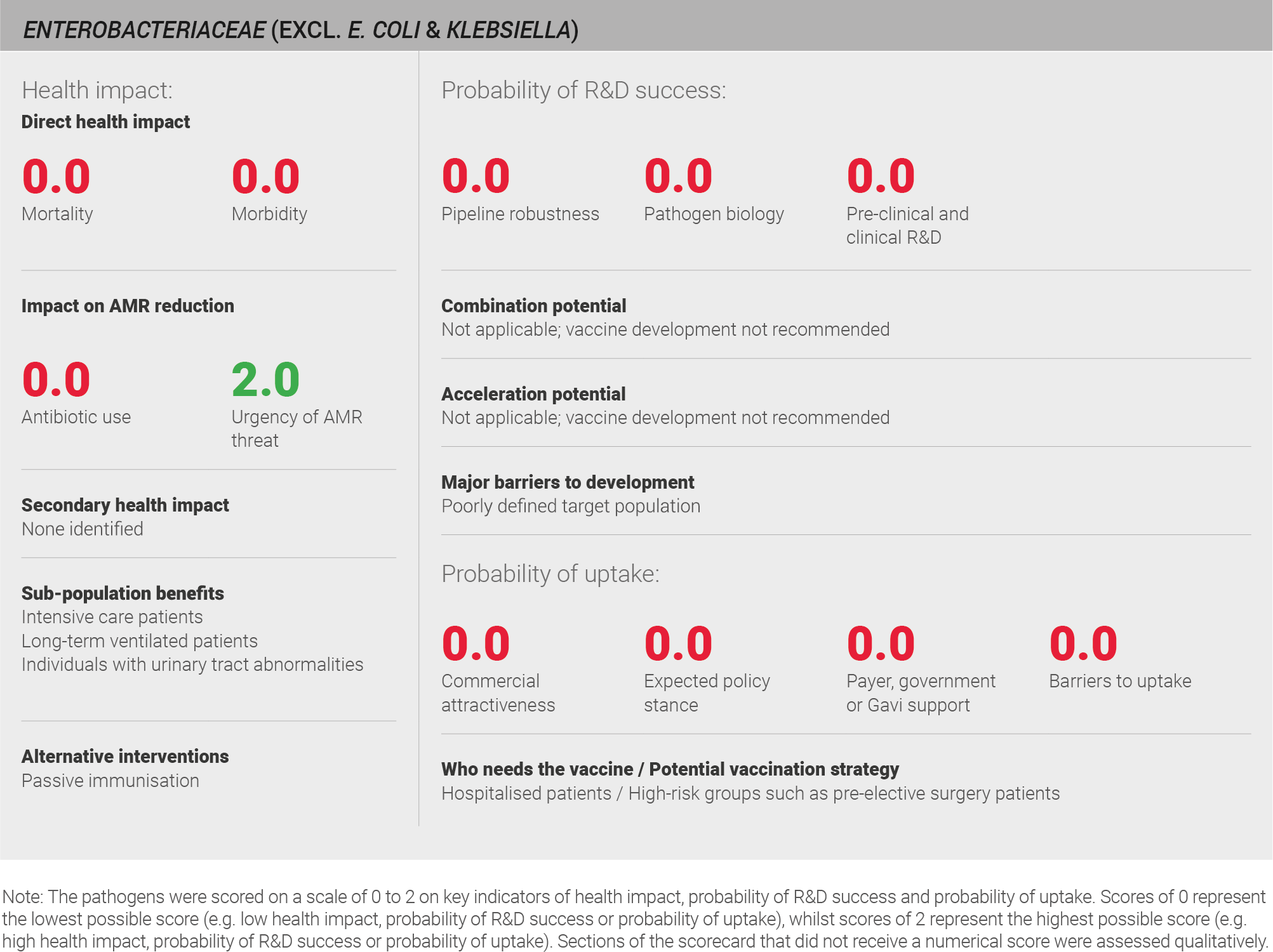
The incidence of Enterobacteriaceae infections is low, at fewer than 10 million cases per year, but the urgency of the AMR threat is high. The current understanding of pathogen biology is poor and vaccine development could be further complicated by the commensal nature of Enterobacteriaceae. The low incidence for each pathogen within the Enterobacteriaceae family means that even a targeted vaccination strategy would not be cost-effective and therefore vaccination is not likely to be recommended by policy bodies.
Enterobacteriaceae falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to explore alternative treatments or prevention strategies. The secondary recommendation is to conduct additional studies in order to better understand the burden, epidemiology and transmission.
Enterobacteriaceae are a family of Gram-negative bacteria that predominantly cause hospital-acquired infections. Enterobacteriaceae family members included on the WHO priority pathogens list and considered as a group are:
- K. pneumoniae
- Escherichia coli
- Enterobacter spp.
- Serratia spp.
- Proteus spp.
- Providencia spp.
- Morganella spp.
Escherichia coli (E. coli) and K. pneumoniae are considered in separate chapters because the higher burden of disease caused by each of these family members merits an individual assessment. Additionally, the Enterobacteriaceae family also includes Salmonella and Shigella, but these are listed as separate pathogens on the WHO priority pathogen list and also evaluated in separate chapters. When the term Enterobacteriaceae is used in this chapter, it is used to refer to the family members above excluding K. pneumoniae and E. coli.
Enterobacteriaceae are commensals that are part of the normal gut flora 83. Urinary tract infections are the most common clinical presentation, but there is a large diversity of clinical syndromes 84 85 86 87 88. These syndromes are associated with the following symptoms:
- Urinary tract infections: dysuria, turbid urine, leakage around catheter, pyrexia, tachycardia, tachypnoea 26
- Pneumonia: cough, purulent sputum, shortness of breath, pyrexia, tachypnoea, tachycardia 89
- Surgical site infections: erythema, swelling, tenderness, wound dehiscence 90
- Endocarditis: malaise, pyrexia, rigors, anorexia, weight loss, splinter haemorrhages, Roth spots on fundoscopy, new murmur on auscultation of the praecordium 91
- Meningitis: headache, pyrexia, nuchal rigidity, confusion, lethargy 92
- Septic arthritis: acutely swollen and painful joint with erythema, warmth and restricted movement 93
Patients who are most susceptible to these infections are typically those who are severely unwell and immunocompromised, such as those in an intensive care setting, or those with other specific risk factors such anatomical abnormalities in the urinary tract. Enterobacteriaceae are transmitted through ascension from the gastrointestinal tract in the case of urinary tract infections 86, or through person-to-person transmission, especially in healthcare settings 86,94,95. Current epidemiological data is insufficient to elucidate variations in global burden.
Direct health impact
Complete global data on the disease burden of Enterobacteriaceae is not available from IHME, WHO, or the research literature 31,32. However, a review of the literature suggests that Enterobacteriaceae causes limited disease burden. Globally, the family is responsible for 2% of urinary tract infections 82, 0.9% of lower respiratory tract infection 33, and 0.3% of neonatal sepsis 34. Data on other clinical syndromes associated with Enterobacteriaceae were scarce. Therefore, it is challenging to assess the global burden of Enterobacteriaceae infection with confidence. A full methodology for this assessment can be found in the appendix.
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
Hospitalised patients, especially those in intensive care settings, and particularly patients on ventilators, would be most likely to benefit from a vaccine. Patients with abnormalities in urinary tract anatomy would also benefit.
Antibiotic use
Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles. Regimens vary in length but a typical regimen involves at least one week of a broad spectrum antibiotic 26,96.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score of 0 out of 2). This estimate is based on an annual incidence of ~ seven million UTIs and ~ three million LRTIs, both treated with a seven day course of antibiotics
Urgency of AMR threat
Both the WHO and CDC have expressed strong concern about antibiotic treatments for Enterobacteriaceae infections. The family is listed as ‘critical’ in the WHO priority list of R&D for new antibiotics 31, carbapenem-resistant Enterobacteriaceae are listed as an ‘urgent’ threat in the CDC’s list of biggest threats from AMR 7, and extended-spectrum beta lactamase-resistant Enterobacterioceae are listed as a ‘serious’ threat by the CDC 7.
Carbapenem-resistant Enterobacteriaceae have been reported across the world and carry a relatively poor prognosis 97. The optimal treatment for these pathogens is uncertain, but these strains frequently require last-line therapies such as polymyxins or combination therapies such as ceftazidime/avibactam or meropenem-vaborbactam 97.
Based on the above analysis, the urgency of AMR threat was categorised as high (score of 2 out of 2).
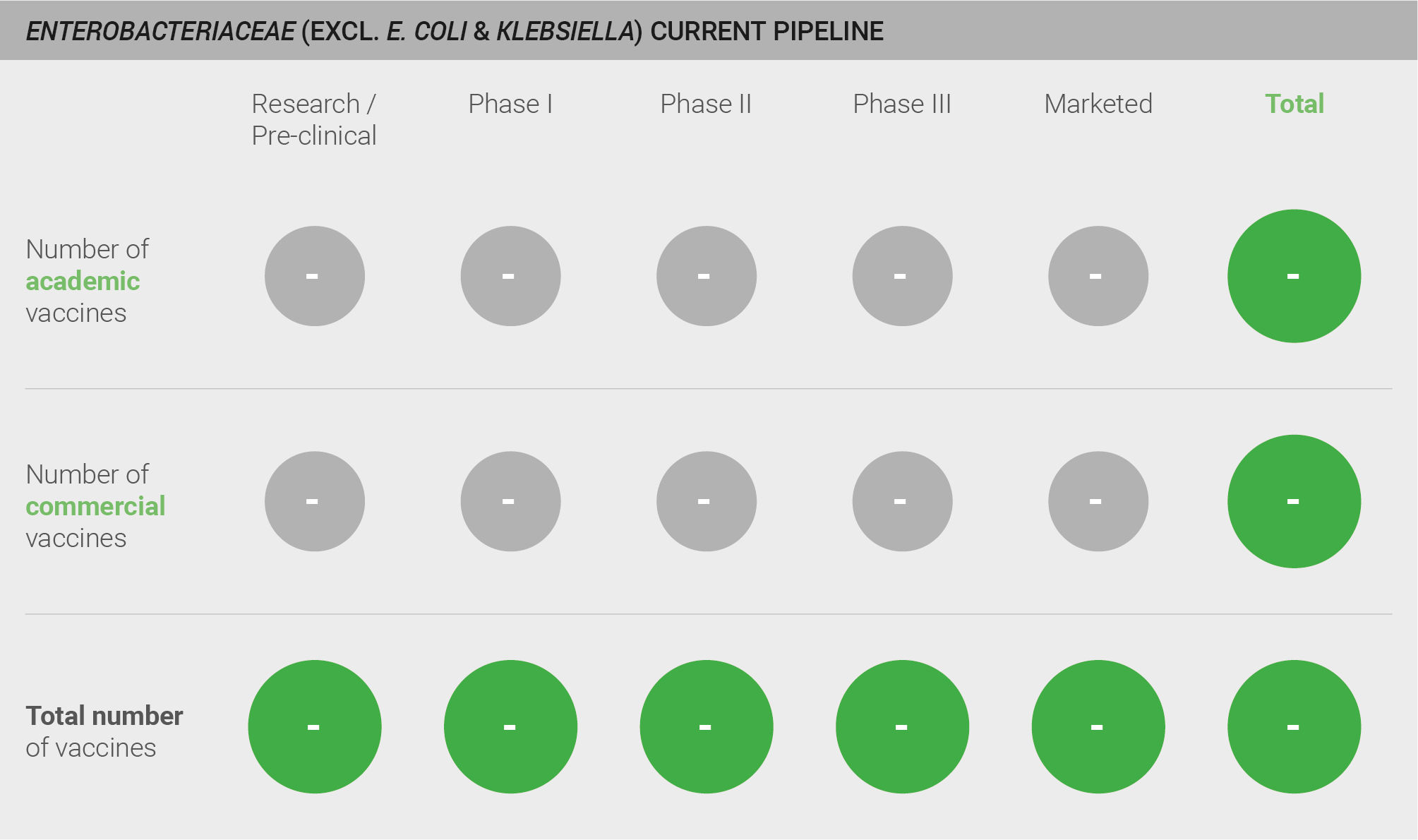
No known candidate vaccines for Enterobacteriaceae are in pre-clinical or clinical development
Scoring: Based on the above analysis, the pipeline was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
Data regarding natural immunity for Enterobacteriaceae are scarce. The case fatality rate is high and if patients recover from infection (for example, those whose risk was due to stressors from surgery or temporary illness) it is difficult to recreate conditions that predispose patients to infection. Based on data for K. pneumoniae and urinary E. coli, it appears unlikely that patients infected with these pathogens develop natural immunity to other Enterobacteriaceae family 98,99.
Proteus is the best-characterised member of the Enterobacteriaceae family included in this analysis. Over 20 outer membrane antigens have been identified in mouse models that are immunogenic and expressed in vivo 100. However, the genus includes a variety of strains, potentially rendering vaccine development difficult 101. Potential targets have also been identified for Serratia. K-antigens and O-antigens could form the basis of antigens for vaccine development and 28 antigens belonging to these groups have been identified 102. However, it is not yet clear from the research literature what proportion of these antigens are sufficiently conserved across strains to render them useful for vaccine development. It is also not yet clear whether these antigens would be immunogenic or whether they are expressed in vivo.
Research into other members of the Enterobacteriaceae family is similarly limited, and strain variability presents a challenge for other family members. Over 30 O-antigens have been characterised for Providencia, suggesting high antigenic variety 103, and a variety of strains exist, likely presenting challenges to vaccine development 101. In silico work has suggested potential vaccine targets for one strain 104. Enterobacter and Morganella also show high strain variety 105,106. Some initial research has also been conducted to delineate virulence factors in Morganella using genomics 107.
Scoring: Based on the above analysis, pathogen biology was categorised as low (score of 0 out of 2).
Pre-clinical and clinical R&D
Although there are animal models of catheter associated UTI and critical care infection 108–110 these models have limitations and there has been very little research using animal models to explore these syndromes when caused by Enterobacteriaceae.
Clinical studies of vaccines targeting Enterobacteriaceae would be very challenging to design and conduct. The low incidence of infections would make achieving adequate trial enrolment challenging. If a targeted vaccine strategy was chosen focusing only on high-risk populations, inducing a protective immune response would be complicated given the compromised state of the patients’ immune systems. The commensal nature of Enterobacteriaceae could cause additional difficulty in development, with possible need to examine impact on patient microbiomes.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as low (score of 0 out of 2).
Expected policy stance
Patients with ICU stays who are at risk of mechanical ventilation or patients with predisposition to urinary tract infection from long term catheterisation or anatomical abnormalities 100 are the populations most likely to benefit from a vaccine targeting Enterobacteriaceae. However, developing a strategy to vaccinate these populations would be extremely difficult. Identifying patients at risk of Enterobacteriaceae infection in ICUs is a major challenge. It is difficult to predict the risk of ICU admission or ventilation in the general population, meaning that a substantive number of patients could not be identified in time to vaccinate and generate a response prior to being at risk of infection. Although there may be cohorts of patients undergoing major elective surgery and with predisposition to UTIs, individual risk of infection from any of the constituent pathogens within the Enterobacteriaceae family is low, hence morbidity and mortality are low in absolute terms.
Policymakers are unlikely to support vaccination for Enterobacteriaceae, primarily because incidence is low individually among pathogen family members and in aggregate. One expert states “none of these [are] of any interest for vaccines. Either target population is too small or you can’t identify target population…we can’t justify vaccinating everybody” 28.
Scoring: Based on the above analysis, expected policy stance was categorised as low (score of 0 out of 2).
Payer, government, or Gavi support
Due to the low incidence and morbidity, the cost-effectiveness for any individual pathogen in the Enterobacteriaceae family is likely to be low. Payers in high-income countries are unlikely to support a vaccine on this basis. Similarly, middle-income countries are unlikely to support vaccines targeting Enterobacteriaceae because their cost-effectiveness thresholds are more stringent than those in high-income countries.
Mortality in Gavi-eligible countries is unknown and unlikely to be higher than in high-income countries. Gavi is unlikely to invest in a vaccine for Enterobacteriaceae given the low absolute mortality burden, therefore, support for a vaccine in low-income countries is likely to be low.
Scoring: Based on the above analysis, payer, government, or Gavi support was categorised as low (score of 0 out of 2).
Barriers to uptake
As discussed in earlier sections, the target population for Enterobacteriaceae vaccines would be difficult to define. For certain populations – for example, those undergoing major elective surgery – vaccination would need to be added to the pre-surgery care bundle. For other populations such as those with long-term catheterisation or anatomical abnormalities elevating the risk of urinary infection, a vaccination touchpoint would have to be instituted at diagnosis or peri-procedure. Patient education would likely be required given that a novel vaccine strategy would be implemented in adults. Finally, if the vaccine is approved for use in new populations, there would be a continued need for dialogue between manufacturers, guideline-setting bodies, and specialist societies to publicise ability to treat pathogens with the vaccine in these new populations.
Scoring: Based on the above analysis, barriers to uptake was categorised as high (score of 0 out of 2).
Commercial attractiveness
The commercial attractiveness of vaccines targeting Enterobacteriaceae is low because of the low incidence of infection, the low mortality of Enterobacteriaceae infections, and the challenges defining a target population.
Scoring: Based on the above analysis, commercial attractiveness was categorised as low (score of 0 out of 2).
Primary recommendation
The primary recommendation is to explore alternatives to vaccination. Given the low incidence of Enterobacteriaceae infections and difficulty in predicting which patients would most benefit from vaccination, passive immunisation represents an alternative strategy for the treatment of Enterobacteriaceae infections. Patients can receive monoclonal antibodies urgently or emergently, and have rapid protection against infection, which lasts for several weeks, obviating the need for preselection and risk stratification. It is likely that development of monoclonal antibodies against Enterobacteriaceae would require further study of pathogen biology in order to identify potential targets for antibodies, given that little is currently known about pathogens in this group. However, expert interviews have suggested that due to strain diversity, one monoclonal antibody may be insufficient to treat all strains of any individual pathogen within the family of Enterobacteriaceae, adding complexity to development of treatments 28. Monoclonal antibody approaches face many of the same development challenges as vaccines.
Some limited early-stage work has explored bacteriophages as a potential treatment for Enterobacteriaceae infections. In vitro work using a biofilm model shows efficacy of a phage cocktail against Proteus 111. Phage therapy offers some potential benefits: it is more specific than antibiotics and therefore less likely to alter the microbiome, with lower risk of drug interactions and toxicities, and retained activity in the presence of biofilms 54,55. The principal disadvantage of bacteriophage therapy is that it requires more personalisation than antibiotics, vaccination or monoclonal antibodies, increasing the expense of treatment, and decreasing scalability 54. Other risks include rapid release of endotoxin (which some Enterobacteriaceae produce 112,113) and risk of transduction of genetic material into the microbiome.
Secondary recommendation
Better characterisation of the disease burden in developed countries attributable to each clinical syndrome would be useful to more accurately assess burden of disease for Enterobacteriaceae; however, collective opinion from expert interviews suggests that further study on global burden would be useful but unlikely to change R&D interest or policy direction. Infections caused by Enterobacteriaceae do not receive policy attention in low-income countries resulting in a lack of studies examining incidence, morbidity and mortality from these pathogens. While one study from Kilifi suggests presence of some of the Enterobacteriaceae family in low-income country settings 58, the disease burden is unlikely to be higher than in high-income countries, and consequently further study is likely to confirm that this pathogen grouping would likely not be prioritised for vaccine R&D.