Acinetobacter baumannii
Acinetobacter baumannii (A. baumannii) primarily causes hospital-acquired infections affecting patients in intensive care settings. It commonly presents as pneumonia. While there is a high urgency of AMR threat, mortality and morbidity are low. The probability of R&D success is low because oth pathogen biology and host immunity are poorly understood. A vaccine targeting patients within intensive care settings is complicated by the difficulty in predicting admission and the immunocompromised nature of the population. There would be significant hurdles to vaccine uptake and vaccination is unlikely to be cost-effective given the low incidence of infection.
A. baumannii falls into a cluster of pathogens for which collecting data and exploring alternatives to vaccination are the priority. The primary recommendation is to explore alternative treatments or prevention strategies, including passive immunisation strategies and bacteriophages. The secondary recommendation is to conduct additional studies in order to better understand the global disease burden.
A. baumannii is a Gram-negative bacterium 23. A. baumannii is ubiquitous and occurs mostly as a commensal pathogen on the skin but is also found in soil, water and plants. It is predominantly hospital-acquired and usually affects patients in the intensive care setting. Clinical manifestations vary by infection site and include:
- Pneumonia, manifesting as dyspnoea, fever, tachypnoea, increased or purulent secretions, haemoptysis, reduced breath sounds, or bronchospasm 24,25. Pneumonia is usually ventilator-associated, but A. baumannii can also rarely cause community-acquired pneumonia 24
- Central line associated infection, manifesting as erythema, and swelling around line or recent line insertion, pyrexia, tachycardia, tachypnoea, or malaise
- Surgical site infections, manifesting as erythema, pain, swelling, or wound dehiscence 24
- Catheter associated urinary tract infections, manifesting as cloudy urine, leakage around catheter, pyrexia, tachycardia, tachypnoea, or malaise 24,26
A. baumannii is transmitted through person-to-person contact such as from the hands of healthcare workers or contact with contaminated medical equipment. In addition, airborne transmission also likely occurs 27. Populations at greatest risk of contracting A. baumannii infection are patients in the intensive care setting or on mechanical ventilation and neonates in India and South East Asia 28.
A. baumannii is widely distributed geographically but the type of infection varies by region. Hospital-acquired infections are reported in Europe, North America, Asia, and the Middle East. In addition, it is a rare cause of community-acquired infections in South Asia, Australia, and the Pacific Islands 24,29,30 Expert interviews suggest that the mortality rate caused by A. baumannii in low-income countries is likely similar to that in high-income countries.
Direct health impact
Global data on disease burden is not directly available from the IHME, WHO, or research literature 31,32 However, research literature suggests A. baumannii causes 1% of lower respiratory tract infections 33. For other clinical syndromes, there is insufficient data on disease burden caused by A. baumannii, or insufficient cases to draw a conclusion about the burden of disease caused by A. baumannii 34,35. Expert interviews suggest that mortality may be underestimated because of the lack of high-quality data regarding neonatal sepsis which is a significant contributor to overall mortality rates. One expert states “[Lack of data is] something we struggle with continually…there are no good, broad studies that help you identify the disease burden” 28 and another calls out the lack of current estimates for neonatal deaths from primary sepsis as a notable inaccuracy, stating, “[the estimates do] not account for sepsis [and] neonatal deaths [from Acinetobacter infection] are primary sepsis” 28. Experts also acknowledge that whilst the best quality data is from high-income countries, they believe the neonatal sepsis burden is highest in India 28. Therefore, the level of confidence in these estimates is relatively low. A full methodology for these estimates is provided in the appendix.
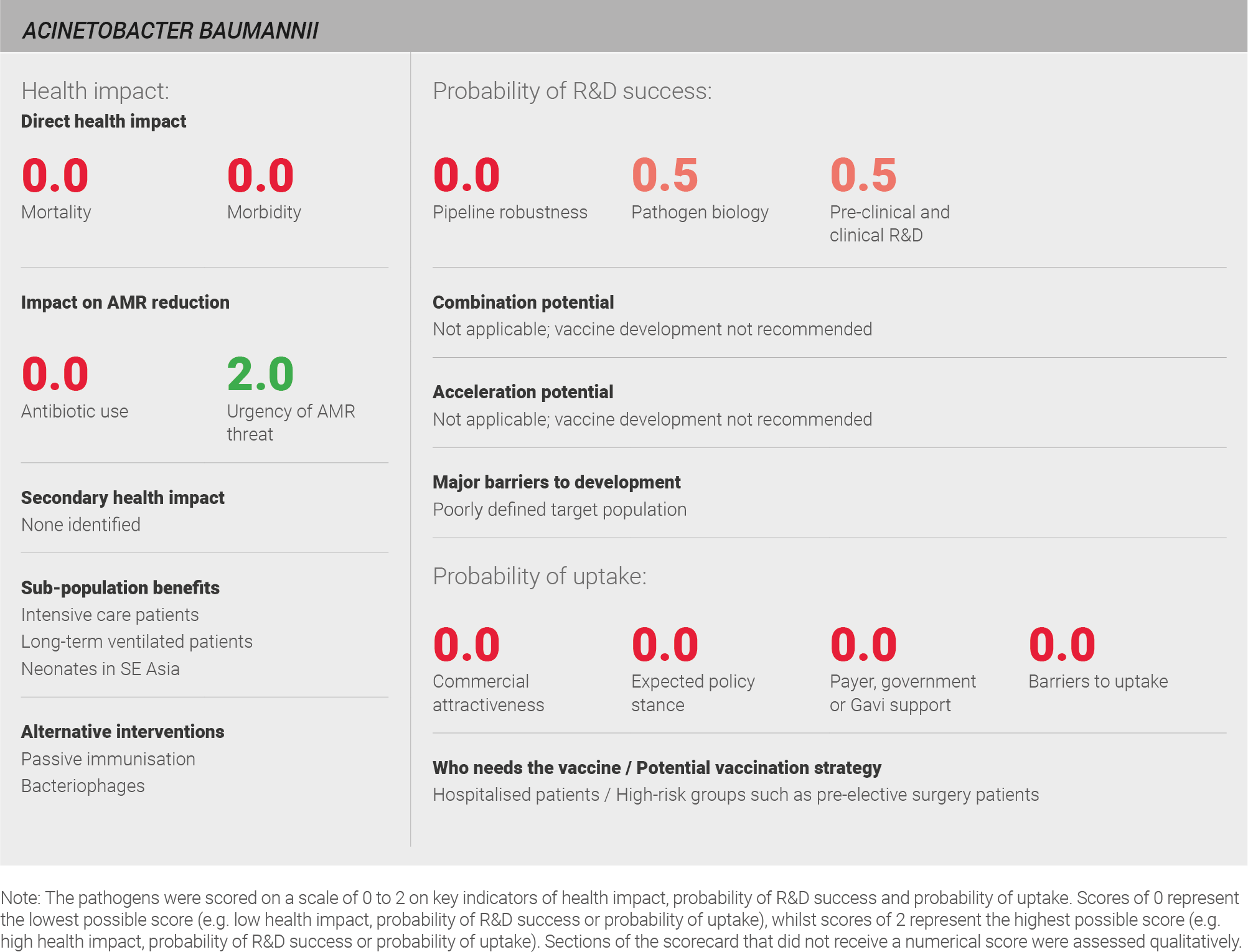
Scoring: Based on the above analysis, mortality was categorised as low (score of 0 out of 2) and morbidity was categorised as low (score of 0 out of 2).
Sub-population benefits
Intensive care patients, patients who rely on mechanical ventilation, and neonates in South East Asia, the groups at highest risk of A. baumannii infection, would benefit the most from vaccination.
Antibiotic use
Recommended antibiotic treatment regimens differ by country, in part reflecting local resistance profiles. Regimens vary in length but a typical regimen consists of a week or more of intravenous broad-spectrum antibiotics. However, given that A. baumannii infection is rarely diagnosed and doctors prescribe treatment based on clinical presentation, it is difficult to know what percentage of cases are treated in accordance with this standard.
Scoring: Based on the above analysis, antibiotic use was categorised as low (score of 0 out of 2). This estimate is based on the relatively low annual incidence of ~three million LRTIs treated with a one week course of antibiotics.
Urgency of AMR threat
Both the WHO and CDC have expressed strong concern about antibiotic resistance developed by A. baumannii. The WHO has listed A. baumannii as a critical priority for R&D regarding new antibiotics 32 and the CDC has listed A. baumannii as a serious threat in its list of greatest threats from AMR 7. The incidence of multi-resistant and extensively resistant strains is rising 36. Multiple untreatable pan-resistant strains have been reported in intensive care and paediatric patient groups 37–39.
Based on the above analysis, the urgency of AMR was categorised as high (score of 2 out of 2).
Pipeline robustness
The pipeline for A. baumannii vaccine development is empty. There are no clinical or pre-clinical vaccine candidates that have been identified in the pipeline analysis, which includes candidates listed in commercial databases or in recent high impact literature reviews40–42.
Scoring: Based on the above analysis, the pipeline robustness was categorised as low (score of 0 out of 2).
Current pipeline
Pathogen biology
The low incidence of A. baumannii infection has precluded an understanding of natural immunity to date 28, and very little is known about host defence mechanisms. A mouse model suggests that mice that have recovered from a previous A. baumannii infection remain susceptible to reinfection 43.
There is somewhat promising, early pre-clinical work in mouse models on vaccine target development and several potential antigens and approaches to developing a vaccine against A. baumannii have been identified. However, it is not known how viable these targets will be in humans. These targets include:
- Formalin inactivated whole cells 44: pre-clinical testing of inactivated whole cells generates robust antibody titres and high survival rates in experiments with mice, suggesting that these vaccines produce functional immunity
- Outer membrane vesicles 45: baumannii secretes outer membrane vesicles which interact with host cells
- Protein-based vaccines including:
- Recombinant outer membrane protein A of baumannii 44: experiments with a mouse model suggests that a vaccine using OmpA would confer protection
- Recombinant Bap 44, a subunit of a surface protein of baumannii, has been shown to be associated with biofilm formation
Scoring: Based on the above analysis, pathogen biology was categorised as fairly low (score of 0.5 out of 2).
Pre-clinical and clinical R&D
Mouse models, including models for pneumonia and wound infection, are being used to study the pathogen, but the clinical relevance of these models is unclear 46. Clinical development of a vaccine against A. baumannii would be difficult. The low incidence of disease would make adequately powered late-stage efficacy trials difficult to conduct. Furthermore, A. baumannii infection usually occurs in immunocompromised patients who may not mount an adequate immune response to the vaccine.
Scoring: Based on the above analysis, pre-clinical and clinical R&D was categorised as fairly low (score of 0.5 out of 2).
Expected policy stance
Two distinct populations could benefit from vaccination – patients at high risk of intensive care admission with mechanical ventilation, and neonates. Both populations pose significant challenges for development of a vaccination strategy. Identifying patients at risk of A. baumannii infection is a major challenge due to the inherent unpredictability of urgent and emergency hospitalisation. Techniques to predict which patients may have a higher absolute risk of infection are not currently available, and although there may be a cohort of patients undergoing major elective surgery who might be at high relative risk of infection, the absolute risk remains very low, so vaccination may not be cost-effective. Vaccination at birth in areas with high rates of neonatal sepsis would be challenging as vaccines are not routinely administered this early in life and there is mixed evidence on efficacy of neonatal vaccination 47,48 Maternal vaccination may be possible as long as a live vaccine was not used 47–51.
At a meeting on vaccination in older adults convened by WHO in 2017, A. baumannii was mentioned as a pathogen for which AMR may be a reason to explore developing a vaccine. Despite this, experts did not think a routine vaccination strategy would be feasible and found it difficult to define a target population suitable for vaccination, limiting the likelihood of policy support. As one expert notes “even if the vaccine could be made, who would you give the vaccine to? We struggle to get adults to take influenza vaccine where we have 500,000 deaths every year. We can’t define the population for which it would be cost-effective to give vaccine”
Scoring: Based on the above analysis, expected policy stance was categorised as low (score of 0 out of 2).
Payer, government, or Gavi support
Without persuasive evidence of disease burden, it would be very difficult for payers or government to calculate the cost-effectiveness of vaccination. Therefore, support for vaccination in high-income countries is unlikely based on current data. The same is true for middle-income countries. Furthermore, based on limited current evidence, it is not likely that vaccination would meet middle-income countries’ thresholds for cost-effectiveness. In low-income countries, current data on mortality is very limited, but mortality appears to be relatively low. Therefore, Gavi support is unlikely. However, the disproportionate burden on neonates in Gavi supported countries may encourage Gavi action.
Scoring: Based on the above analysis, payer, government or Gavi support was categorised as low (score of 0 out of 2).
Barriers to uptake
The difficulty in defining the target population for an A. baumannii vaccination presents a major logistical hurdle to vaccination uptake. In the most clear-cut case of vaccinating before planned surgical procedures, the vaccine would need to be incorporated into the pre-surgical care pathway for patients, which would be possible 52. For other potential strategies, new touchpoints would need to be created.
If evidence was obtained for vaccination in new sub-populations, close engagement with guideline setting bodies and specialist societies would be required in order to ensure that licensure was translated into awareness and use of vaccine by clinicians.
Scoring: Based on the above analysis, barriers to uptake were categorised as high (score of 0 out of 2).
Commercial attractiveness
The commercial attractiveness of vaccination for A. baumannii is low. It is difficult to assess market size based on current epidemiological data and to define a well-circumscribed target population.
Scoring: Based on the above analysis, commercial attractiveness was categorised as low (score of 0 out of 2).
Primary recommendation
The primary recommendation is to explore alternative treatments or prevention strategies for diseases caused by A. baumannii. Given the low incidence of A. baumannii infections and difficulty in predicting which patients would most likely benefit from vaccination, passive immunisation with monoclonal antibodies represents an alternative strategy. Patients can receive monoclonal antibodies urgently or emergently, providing rapid protection against infection, which lasts for several weeks, making this approach potentially better-suited to patients in the intensive care setting than conventional vaccines. Initial work in mouse models of infection has demonstrated improved survival using monoclonal antibodies targeting an A. baumannii capsular carbohydrate 53. It is likely that development of monoclonal antibodies against A. baumannii would require further study of pathogen biology in order to identify potential targets for antibodies. Although developing a strategy for passive immunisation would face similar difficulties as developing a strategy for vaccination patients could be more easily targeted with monoclonals given the emergent and often unpredictable nature of infection risk. Bacteriophages may provide another alternative approach to treating A. baumannii infection.
There is a case report of a patient with multidrug resistant A. baumannii who improved after intravenous administration of bacteriophages. The bacteriophage was selected from a phage library after testing against an A. baumannii culture from the patient 54. Advantages of phage therapy are that it is more specific than antibiotics, so less likely to alter the microbiome, with lower risk of drug interactions and toxicities, and retained activity in the presence of biofilms 54,55 The principal disadvantage of bacteriophage therapy is that it requires more personalisation than antibiotics, vaccination or monoclonal antibodies, increasing the expense of treatment, and decreasing scalability 54. Other disadvantages include the risk of rapid release of endotoxin (which A. baumannii produces) from bacterial cell lysis and risk of transduction of genetic material into the microbiome 55. Experts convey that early data on bacteriophages appeared promising but that there are outstanding issues; bacteriophages are often very strain-specific and cannot infect and lyse all strains 28. They also cite some concern about immune reactions to bacteriophages 28.
Secondary recommendation
A better understanding of the disease burden, epidemiology, and transmission of A. baumannii is needed. Studies of hospital-acquired pneumonia and ventilator acquired pneumonia have identified variable rates of A. baumannii infection (~5%- ~20% for hospital-acquired pneumonia and ~4%-~40% for ventilator acquired pneumonia 56). A. baumannii has also been reported to cause surgical site infections 57,58. Estimating the global burden of disease is particularly difficult given the paucity of data from low- and middle-income countries. In light of the apparent variability in infection rates, it would be useful to further characterise disease burden through multisite studies to understand within country variability in burden and through multi-country studies to understand the global burden. However, whilst better characterisation of the burden is needed and case fatality is high, it is unlikely that incidence estimates will change enough for A. baumannii to be prioritised for vaccine development.