Executive Summary
The role of vaccines in combating antimicrobial resistance (AMR)
AMR is a significant and growing problem. Drug resistant infections cause 700,000 deaths per year; this number could rise to 10 million by 2050 1 unless urgent action is taken. Furthermore, this figure does not capture the impact of being unable to safely perform high-risk medical procedures such as complex surgery or chemotherapy.
Immediate and coordinated action is required to tackle the threat posed by AMR. Vaccines alone will not be sufficient to achieve this, but they are critical tools that can play an important role when deployed alongside broader activities. A multi-faceted, One Health approach must be used because the emergence of resistance stems from behaviour across human and animal health. The development of new antibiotics and alternative therapeutics, the rational use of antibiotics in human and animal health, more effective use of diagnostics, improvements to water, sanitation and hygiene, and vaccines can all support efforts to combat AMR.
However, vaccines do have some unique advantages, and therefore bringing additional, and more effective, vaccines to market could have a huge impact on AMR. Vaccines already play a critical role, with an impressive track-record of reducing AMR 2. Both H. influenzae b and S. pneumoniae vaccines have resulted in a dramatic reduction in disease burden and have been associated with decreased incidence of resistant strains. Additionally, both vaccines have an additional “indirect” effect on AMR by reducing antibiotic usage and therefore selection pressure on pathogens. Evidence shows that universal coverage with 13-valent S. pneumoniae vaccination could avoid 11.4 million days of antibiotic use per year in children under five 3.
Vaccines also offer a long-term sustainable approach to infection prevention, because pathogen resistance to vaccines is not common. For example, vaccines against diphtheria and pertussis have been in use for 70 years without resistance developing.
Purpose of this report
This report seeks to provide an independent, actionable assessment of the potential of vaccines to combat AMR, and encourages greater attention, focus, and funding for vaccine development against pathogens whose resistance to antimicrobial medicines was identified by WHO as posing the greatest threat to human health. By employing a carefully considered prioritisation framework to evaluate these pathogens, this report enables comprehensive comparisons across pathogens. This assessment and prioritisation provides a guide for research priorities, policy focus and investment decisions, while recognising that individuals and institutions have varied areas of focus and seek to interact at different parts of the value chain. Additionally, this report consolidates information on these pathogens, and on the development efforts against them, which is currently fragmented, providing a critical new resource to the community working to address AMR.
Scope of this report
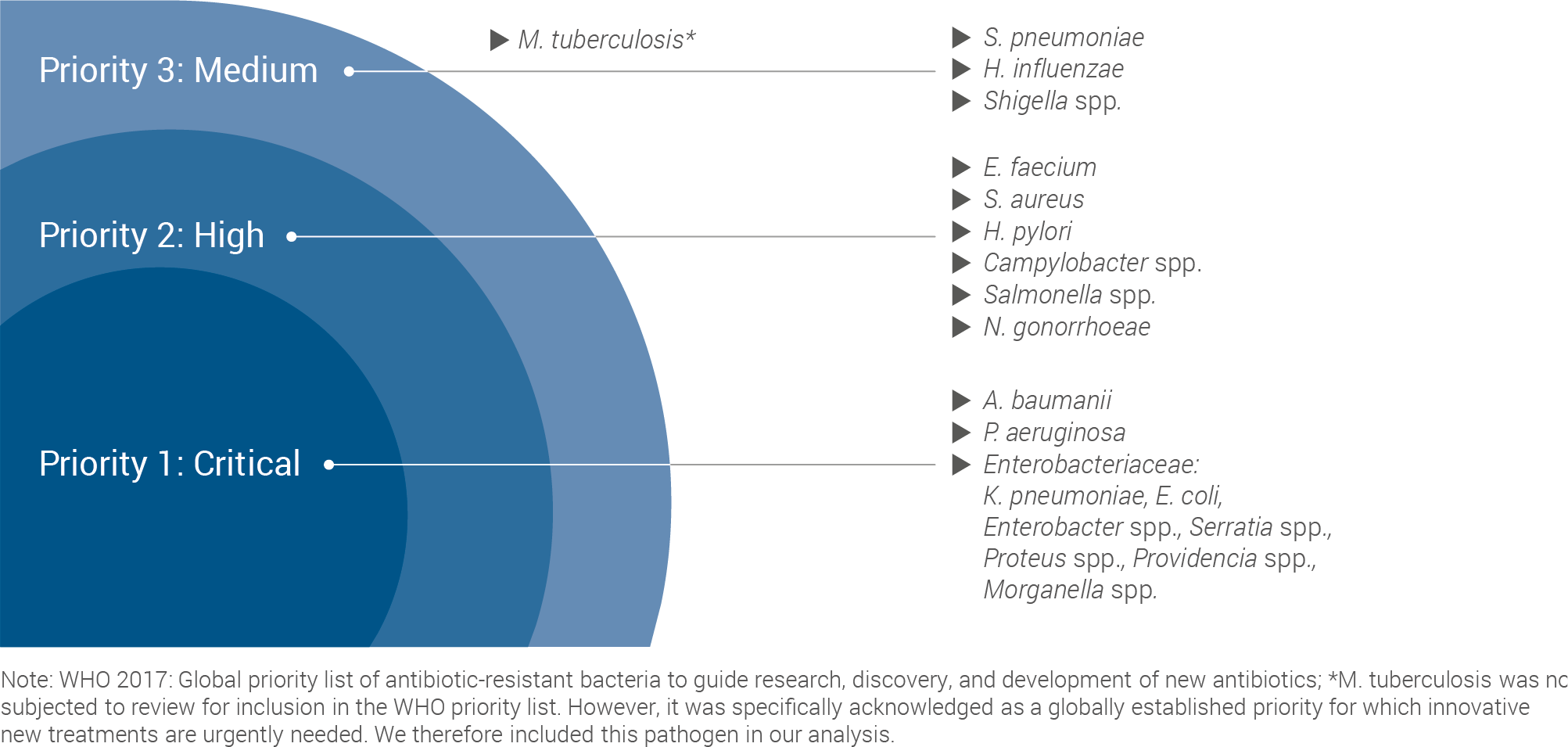
This work used the World Health Organisation (WHO) priority pathogen list as a starting point for the assessment:
WHO LIST PROVIDES STARTING POINT FOR COMPARATIVELY ASSESSING VACCINES FOR PATHOGENS WITH HIGH LEVELS OF AMR
Please click on image for printable version
It is important to note that additional pathogens, such as influenza virus, contribute to inappropriate antibiotic usage and selection for AMR, however they were not included in the assessment at this time. By setting out a robust and durable framework and methodology, this work can be taken further in the future by expanding the comparative set of pathogens and increasing the sophistication of individual metrics and indicators.
Methodology employed to assess pathogens
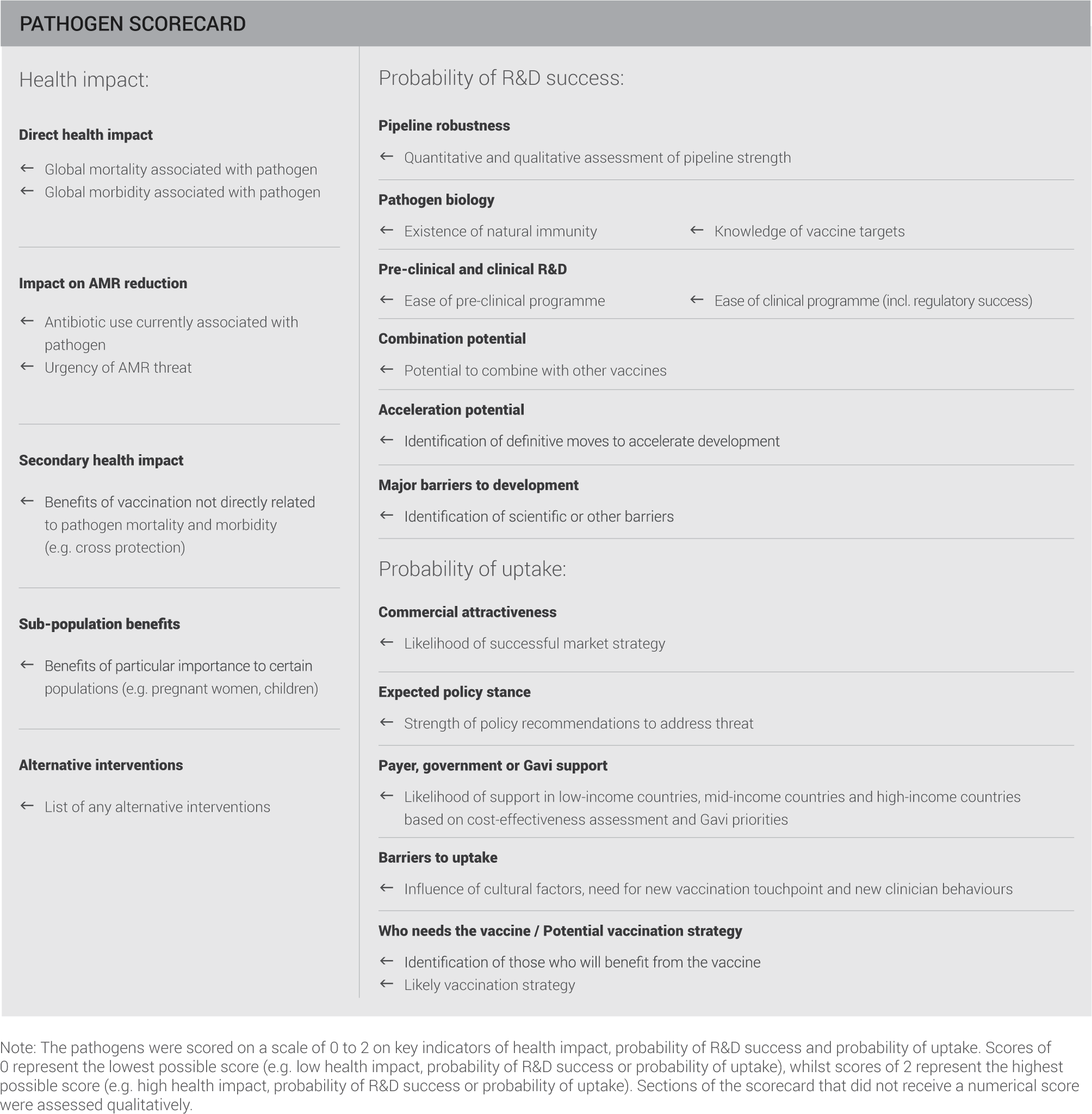
Each pathogen was evaluated based on the potential health impact of a vaccine against the pathogen, the probability of R&D success and the probability of vaccine uptake. The assessment evaluated the development of vaccines against all strains of each pathogen – not just those which are resistant to antibiotics.
This evaluation included the criteria and indicators summarised on the scorecard:
PATHOGENS ASSESSED ACCORDING TO HEALTH IMPACT, PROBABILITY OF R&D SUCCESS AND PROBABILITY OF UPTAKE
Please click on image for printable version
This scorecard assessment aims to holistically capture the stages of vaccine development and establishing a vaccination programme.
Determining the health impact of a pathogen provides critical information, both for those looking to develop vaccines and those responsible for establishing and funding vaccination programmes. In the context of AMR, an understanding of the direct health impact of a pathogen needs to be assessed alongside the level of AMR threat the pathogen causes and the degree to which it drives antibiotic use.
Once a case for vaccine development is established it is important to understand how feasible the development process is likely to be. The robustness of the current pipeline provides a useful indication of the ease of advancing vaccine candidates and the level of commercial interest. A more detailed understanding of potential challenges is gained by assessing pathogen biology and the ease of pre-clinical and clinical programmes.
Assuming a vaccine is successfully brought to market, a key question remains about the likelihood of implementing a successful vaccination programme. A wide range of bodies will influence this process, including policy makers, payers and international organisations such as, the WHO, Gavi and UNICEF – the relative importance of these will vary by pathogen. Finally, it is important to identify any significant barriers that could prevent uptake.
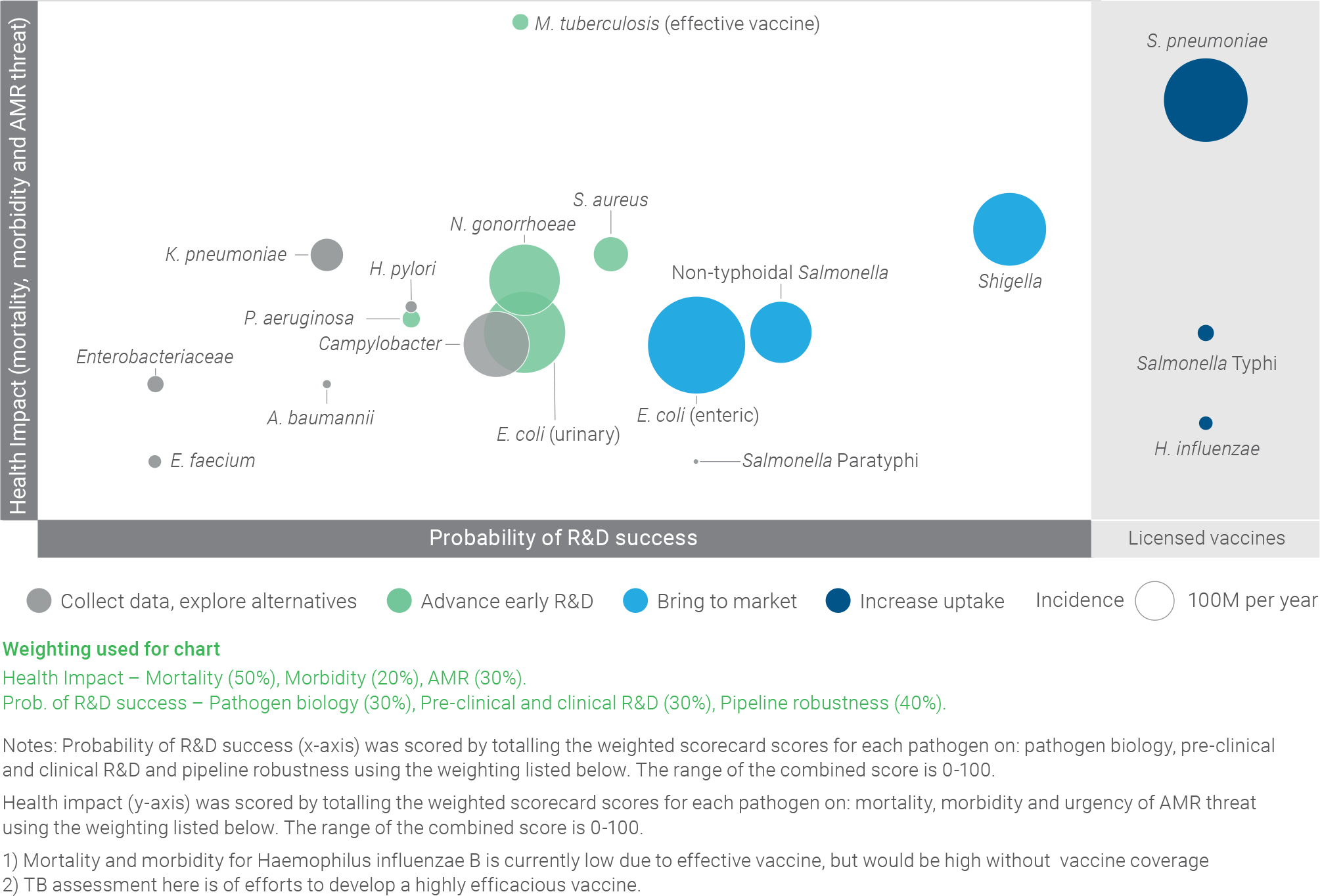
Pathogen clusters identified through this assessment
This assessment resulted in the identification of pathogen clusters that can help prioritise interventions, as illustrated in the figure below:
- The “increase uptake” cluster (dark blue) is composed of pathogens with effective, marketed vaccines where the key recommendation is to increase uptake
- The “bring to market” cluster (light blue) is composed of pathogens with significant health impact and sufficiently advanced R&D to recommend concentrating on accelerating vaccines through clinical development to market
- The “advance early R&D” cluster (green) is composed of pathogens with significant health impact, where more investment in early-stage R&D is needed to develop and advance a robust pipeline of vaccine candidates
The “collect data, explore alternatives” cluster (grey) is composed of pathogens that are less well-suited to vaccine development, as well as pathogens where more information is needed to determine whether vaccine development should be a priority
PATHOGEN SEGMENTATION BASED ON ASSESSMENT CREATES CLUSTERS THAT CAN HELP PRIORITISE INTERVENTIONS
Please click on image for printable version
Each pathogen falls within its cluster for a set of different reasons. It is therefore important to understand each pathogen in addition to its cluster when prioritising efforts. A summary for each pathogen is included below. A full discussion of this matrix is included in the appendix.
Pathogen clusters
Increase uptake for existing, effective vaccines
Pathogens on the WHO list with effective vaccines include H. influenzae, S. pneumoniae and S. Typhi:
- Although uptake of H. influenzae vaccine is relatively high globally at ~70%, continued efforts can be made to maintain and further expand coverage, particularly in certain geographies.
- Increasing uptake of the S. pneumoniae vaccine presents a significant opportunity; this vaccine is effective for 13 serotypes and used in high, middle and low-income countries, but currently only has ~40% coverage.
- A new, conjugated S. Typhi vaccine has recently been pre-qualified by the WHO and is supported by Gavi for introduction in 2019, following effectiveness trials. Upon completion, efforts should focus on successfully introducing a vaccination programme.
Bring to market new vaccines for pathogens where protective immunity is understood, by accelerating clinical development
Pathogens on the WHO list in this category include E. coli (enteric), non-typhoidal Salmonella and Shigella:
- The high antigenic diversity of E. coli (enteric) is a challenge for vaccine development, but inclusion of LT toxoid and fimbrial antigens in a potential vaccine may help cover 70-80% of strains.
- A non-typhoidal Salmonella vaccine appears technically promising and potentially impactful, given high disease burden in Africa.
- A vaccine against Shigella would represent a major opportunity in this segment due to high incidence and significant associated mortality, particularly in low- and middle-income countries.
Advance early R&D for high impact pathogens with unclear R&D feasibility, by investing in early stage research
Pathogens on the WHO list in this category include M. tuberculosis (due to sub-optimal effectiveness of BCG vaccine), N. gonorrhoeae, P. aeruginosa, S. aureus and E. coli (urinary):
- There is a strong case for vaccine development for M. tuberculosis given its health impact and AMR threat. However, current difficulties in understanding pathogen biology and translatability of pre-clinical research must be overcome.
- The case for development of a vaccine targeting N. gonorrhoeae is strong due to high incidence, high morbidity, and current circulation of resistant strains. Although significant development challenges remain, evidence of MenB vaccine cross-protection has fostered fresh optimism in the expert community.
- E. coli (urinary) has a high incidence and would be attractive for targeted vaccination in high-income countries, but antigen selection remains a challenge
- Vaccine development for P. aeruginosa is attractive for high-risk patient groups, such as cystic fibrosis patients, but vaccine development is difficult because the target population is predominantly composed of immunocompromised patients.
- Morbidity and mortality from S. aureus in high-income countries means the market for a vaccine is attractive, with significant commercially-driven activity. However, there are significant gaps in understanding disease burden and identifying vaccine targets and animal models have limited predictive capability.
Collect data and explore alternatives for those pathogens on the list less well-suited to vaccine development due to significant outstanding epidemiological questions, low incidence and associated mortality and morbidity, or preferable alternative strategies
Pathogens on the WHO list that are not currently well-suited to vaccine development: A. baumannii, Campylobacter, E. faecium, Enterobacteriaceae, H. pylori, K. pneumoniae and S. Paratyphi:
- S. Paratyphi has low incidence and low associated mortality and morbidity, consequently, uptake of a standalone vaccine is unlikely. Therefore, the priority should be to explore combination vaccines with S. Typhi.
- More data is needed on Campylobacter transmission in low- and middle-income countries, particularly to understand whether transmission occurs through environmental pathways or from animal reservoirs. This will guide a determination on whether a human vaccine should be pursued or whether alternatives, such as animal vaccination, will be the preferred approach.
- A better understanding of the link between H. pylori and gastric cancer, as well as a better understanding of how AMR is likely to evolve due to relative current treatability of the pathogen, is necessary.
- K. pneumoniae has a higher burden than most other hospital-acquired infections, but more data is needed to help determine whether there are predictable sub-populations to target for clinical development and vaccine delivery. Additionally, further study is needed to more accurately estimate the disease burden.
- Due to the comparatively low incidence, morbidity, and mortality of Enterobacteriaceae, A. baumannii and E. faecium, they are not considered strong candidates for vaccine development. Alternatives, such as passive immunisation, should be explored. Additionally, these pathogens are Gram-negative pathogens that cause hospital-acquired infections in small, immunocompromised target populations. These characteristics present particularly challenging hurdles for vaccine development.
A detailed assessment and recommendations for each pathogen can be found in the individual pathogen chapters.
Recommendations
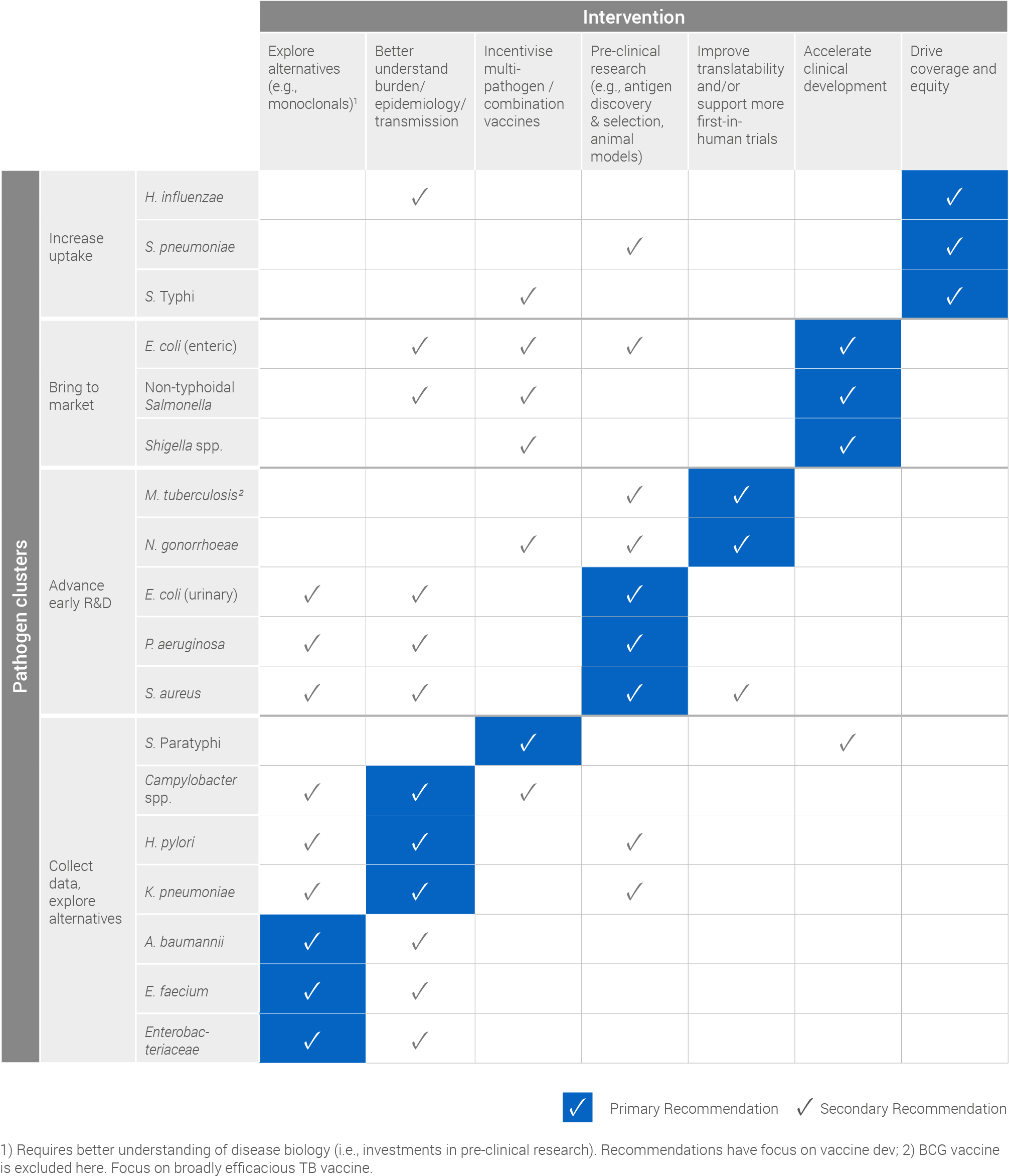
Based on its cluster, each pathogen has a primary, or most critical, recommendation for intervention which has been summarised in the following table. Secondary recommendations, which detail other actions that can help advance vaccine development and / or uptake for each pathogen, have also been included.
SUMMARY OF INTERVENTION RECOMMENDATIONS
Please click on image for printable version
Cross-cutting activities would stimulate development of vaccines for all AMR priority pathogens
Through the process of making detailed recommendations specific to each pathogen, this report also identified knowledge gaps shared across multiple pathogens. Based upon these, several cross-cutting activities have been proposed which, if addressed in a coordinated manner, would stimulate development of vaccines for all pathogens with high levels of AMR.
Health Impact
- Promote the collection of robust epidemiological data, which is currently limited and varies greatly in quality across the pathogen set and is critical to making an investment / business case for vaccine development and for encouraging vaccine uptake.
- Model the evolution of AMR and potential health impact of interventions, potentially through a consortium of modellers, which could serve as a common resource for the global health community
Research and Development
- Target investment to new R&D platforms relevant to AMR pathogens such as DNA and RNA vaccines, viral vectors, nanoparticles, novel delivery/administration technologies, and modular manufacturing platforms. These have the potential to significantly lower vaccine manufacturing costs and to facilitate development of polyvalent vaccines.
- Collaborate for regulatory innovation, including encouraging regulatory acceptance of AMR and antibiotic usage as a measurable outcome and encouraging more regular convenings with the aim of harmonising regulatory processes where possible.
Uptake
- Continue to utilise and improve market shaping interventions where needed, with adaptation to the unique requirements of AMR priority pathogens.
- Develop the health economic case for vaccination which includes the value of a vaccine in combatting AMR, as this is absolutely critical to policy and payer recommendations.
These recommendations are discussed in more detail in the cross-cutting activities section of this report.
Action is needed today to harness these potent tools to tackle AMR
Developing a new vaccine can take decades of intensive R&D without any guarantee of success. Bringing the 13-valent pneumococcal conjugate vaccine to market required 13 clinical trials in nine countries – clearly a considerable undertaking despite decades of knowledge from developing and marketing a 7-valent vaccine 4. Even with significant investment, only ~11% of vaccines being actively developed in 1995 were on the market by 2011 5. With all these challenges, action is required today to ensure a robust pipeline of candidates is developed to target high priority pathogens. Failure to take decisive action now will deprive the global community of a potent tool for tackling AMR.